Final ID: MP2380
Primary Results of the VICTORION-NOVEL (LDL-C maNagement PrOgram in Atherosclerotic Cardiovascular Disease (ASCVD) patients with Elevated LDL-C) Lipid Optimization Multicenter Implementation Trial
Abstract Body (Do not enter title and authors here): Background: Most patients with atherosclerotic cardiovascular disease (ASCVD) do not advance beyond statin therapy and do not achieve the recommended low-density-lipoprotein cholesterol (LDL-C) thresholds. Gamification between peer clinicians may boost performance in lipid management.
Research Question: Can a multifaceted gamified intervention, including a live performance dashboard, peer-to-peer discussions, and clinician and patient engagement materials improve LDL-C optimization in patients with ASCVD?
Methods: VICTORION-NOVEL was a US prospective, multicenter implementation trial including 66 clinicians across ambulatory practices at 6 sites (3 intervention and 3 control) between September 2023 to August 2024. Adults ≥18 years with established ASCVD, statin therapy and LDL-C ≥ 70 mg/dL were included. Primary outcome was the probability of experiencing a composite endpoint, defined as 1) achieving LDL-C < 70 mg/dL, 2) achieving ≥ 20% reduction in LDL-C, or 3) experiencing intensification of lipid lowering therapy (LLT) within 1 year. Extended Cox regression model was used to assess time to earliest composite endpoint, adjusting for confounders.
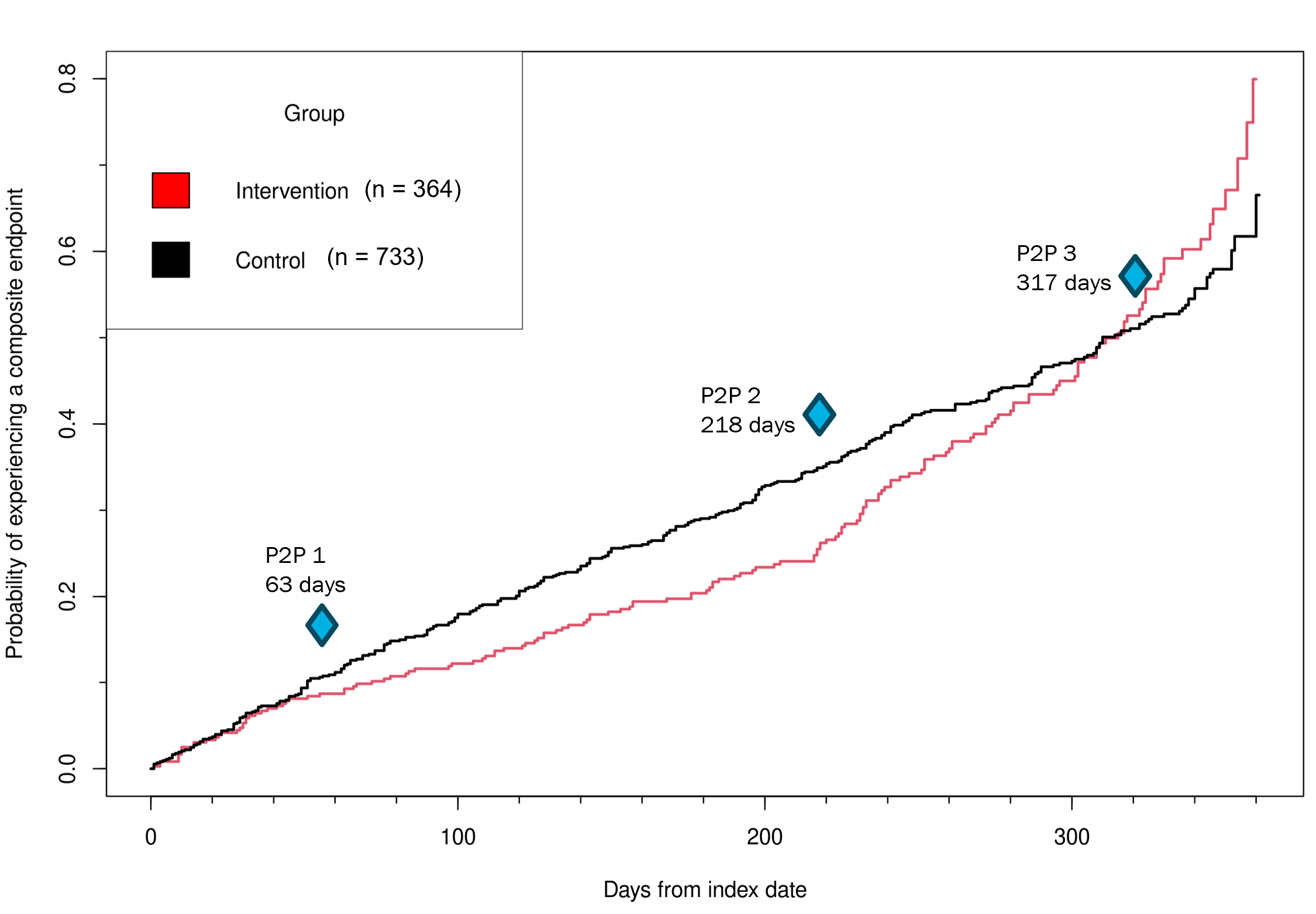
Results: Overall, 1,097 patients (364 intervention, 733 control) were included (mean age 68, 45% women). While age and sex distributions were balanced, patients in the control arm were more likely to be White (89% vs. 79%), non-Hispanic (97% vs. 67%) and less likely to have hypertension (47% vs. 81%) and atrial fibrillation (2% vs. 20%). A significant time-varying effect on the primary endpoint was observed during the 1-year follow-up, with potentially late beneficial effects emerging after the second peer-to-peer call (adjusted HR 1.34; 95% CI 1.07-1.68). At 1-year, a higher proportion of intervention patients achieved LDL-C<70 mg/dL than control patients (23% vs. 16% p<.01), while similar proportions of patients achieved 20% reduction in LDL-C (30% vs. 32%) and had intensification of LLT (17% vs. 22%). The comparative data dashboard, which allowed providers to assess and compare their own patients’ outcomes to the outcomes of their colleagues’ patients, was reported as a helpful tool.
Conclusions: While the implementation of a gamified, multifaceted intervention did not significantly improve overall lipid optimization at 1-year, it was associated with a higher proportion of patients achieving LDL-C goals. The delayed emergence of benefit suggests that a lead-in period may be required for full effectiveness.
Research Question: Can a multifaceted gamified intervention, including a live performance dashboard, peer-to-peer discussions, and clinician and patient engagement materials improve LDL-C optimization in patients with ASCVD?
Methods: VICTORION-NOVEL was a US prospective, multicenter implementation trial including 66 clinicians across ambulatory practices at 6 sites (3 intervention and 3 control) between September 2023 to August 2024. Adults ≥18 years with established ASCVD, statin therapy and LDL-C ≥ 70 mg/dL were included. Primary outcome was the probability of experiencing a composite endpoint, defined as 1) achieving LDL-C < 70 mg/dL, 2) achieving ≥ 20% reduction in LDL-C, or 3) experiencing intensification of lipid lowering therapy (LLT) within 1 year. Extended Cox regression model was used to assess time to earliest composite endpoint, adjusting for confounders.
Results: Overall, 1,097 patients (364 intervention, 733 control) were included (mean age 68, 45% women). While age and sex distributions were balanced, patients in the control arm were more likely to be White (89% vs. 79%), non-Hispanic (97% vs. 67%) and less likely to have hypertension (47% vs. 81%) and atrial fibrillation (2% vs. 20%). A significant time-varying effect on the primary endpoint was observed during the 1-year follow-up, with potentially late beneficial effects emerging after the second peer-to-peer call (adjusted HR 1.34; 95% CI 1.07-1.68). At 1-year, a higher proportion of intervention patients achieved LDL-C<70 mg/dL than control patients (23% vs. 16% p<.01), while similar proportions of patients achieved 20% reduction in LDL-C (30% vs. 32%) and had intensification of LLT (17% vs. 22%). The comparative data dashboard, which allowed providers to assess and compare their own patients’ outcomes to the outcomes of their colleagues’ patients, was reported as a helpful tool.
Conclusions: While the implementation of a gamified, multifaceted intervention did not significantly improve overall lipid optimization at 1-year, it was associated with a higher proportion of patients achieving LDL-C goals. The delayed emergence of benefit suggests that a lead-in period may be required for full effectiveness.
More abstracts on this topic:
Aggressive LDL cholesterol lowering post ACS with triple combination therapy: Insights from the multicentric LAI-REACT study
Puri Raman, Mahajan Kunal, Agarwala Rajeev, Gupta Ashu, Batra Aditya, Khan Aziz, Vijan Vinod, Sharma Jai Bharat, Himral Surender
Acetylation of mitochondrial LCAD and SOD2 promotes metabolic dysfunction, oxidative stress, multi-organ damage and hypertensionDikalov Sergey, Nogueira Marina, Polosukhin Vasiliy, Gius David, Milne Ginger, Dikalova Anna