Final ID: MP2329
Emergence and Applications of FDA-Cleared Artificial Intelligence in Cardiovascular Care
Abstract Body (Do not enter title and authors here): Introduction:
Artificial Intelligence and machine learning (AI/ML) technologies are rapidly expanding in the field of medicine, as well as cardiology. Studies within cardiovascular medicine have examined the unique capabilities, potential, and critical gaps in these devices. However, there is limited review of the types of devices that are being cleared by the U.S. Food and Drug Administration (FDA). The FDA primarily regulates new AI/ML devices, and understanding cleared devices may provide a better understanding of the landscape.
Research Question:
What are the characteristics of AI architecture, clinical indications, and regulatory pathways of devices that receive FDA clearance?
Aims:
This study aims to analyze the trends of FDA clearances of AI/ML-enabled cardiovascular devices, changes in device characteristics, and pre-clearance clinical validation of new devices.
Methods:
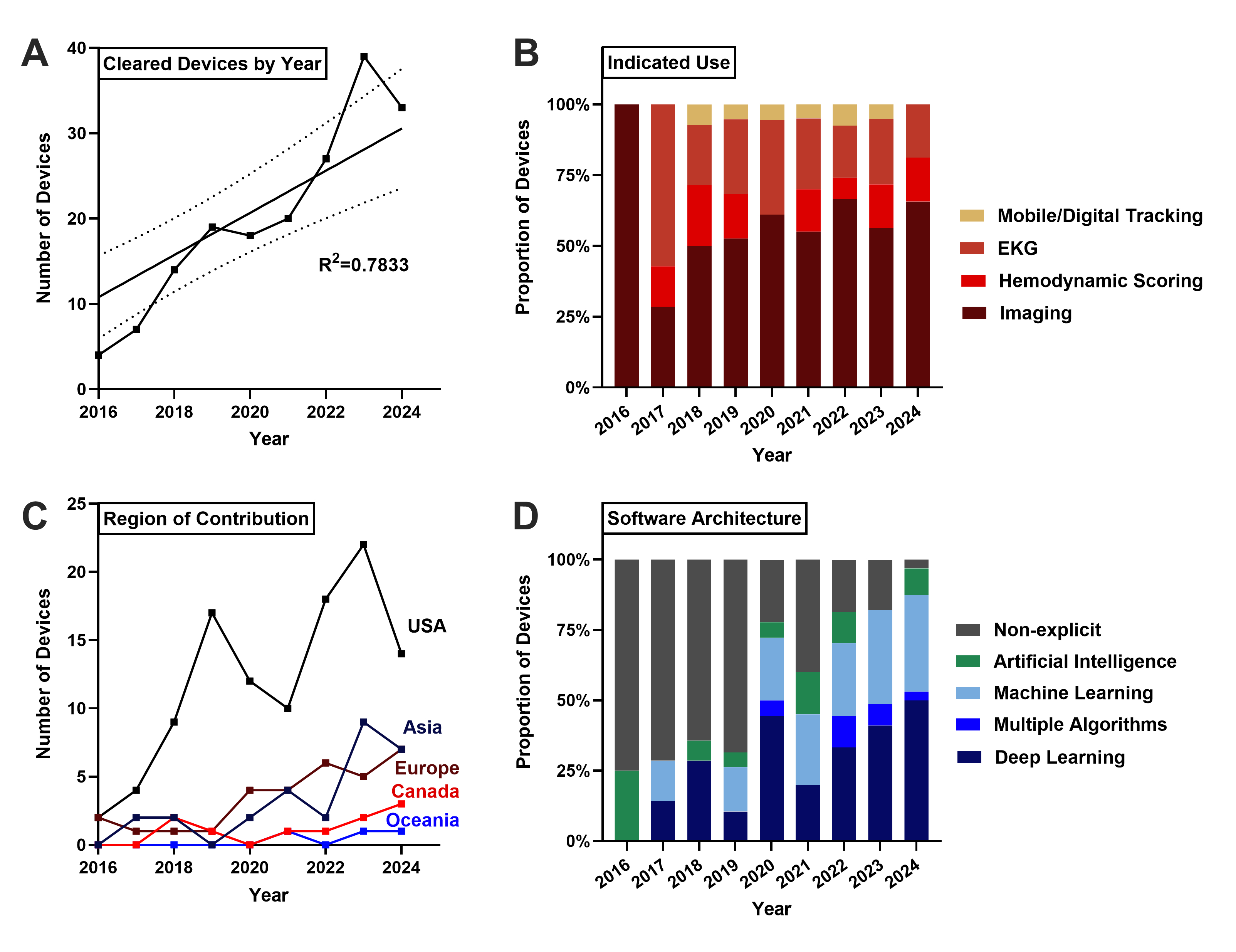
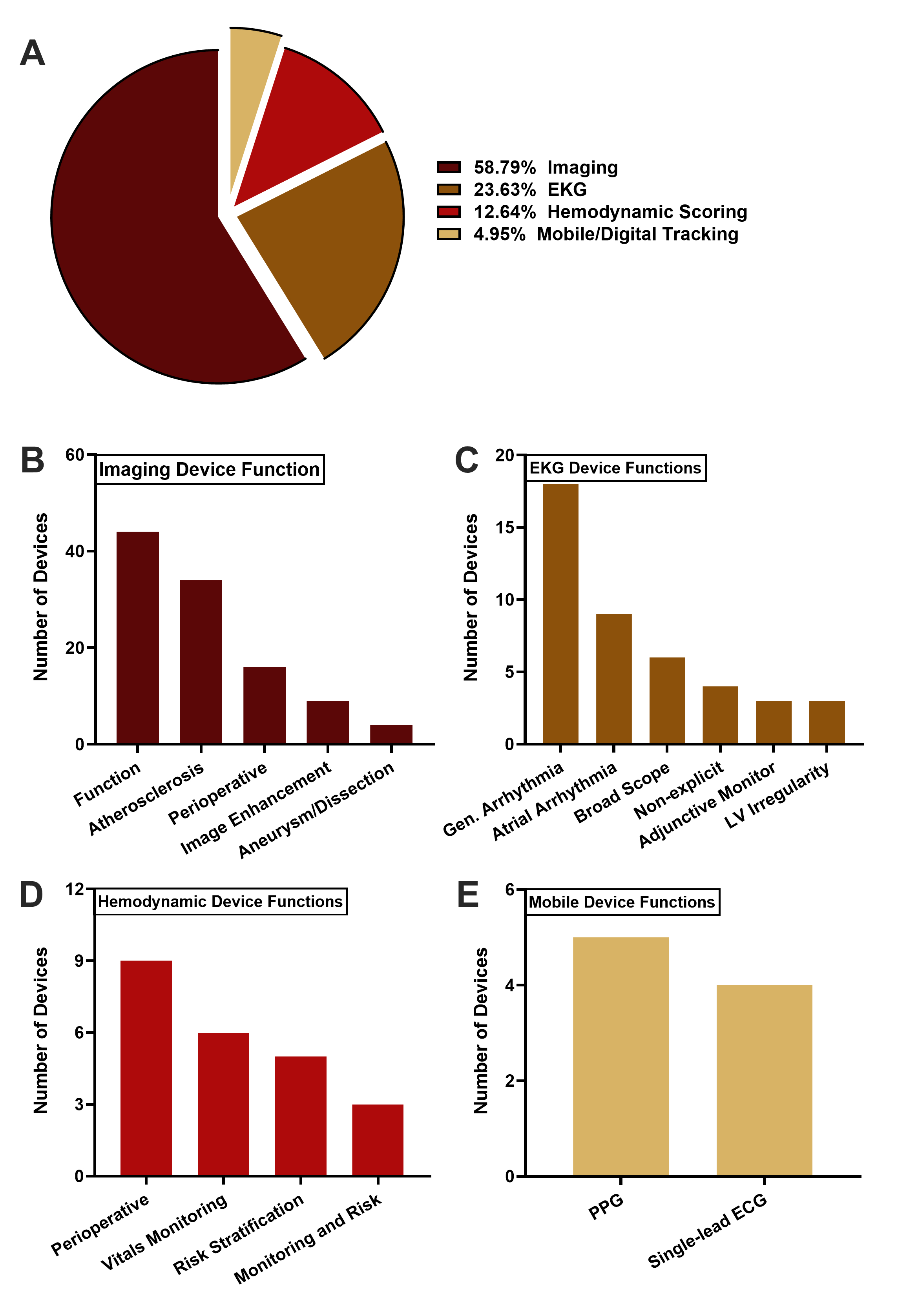
Data was collected from the FDA AI/ML-enabled Medical Devices Database. Of the 1,016 devices cleared, 182 cardiovascular devices were identified. Devices were divided into four clinical indications: Imaging, Electrocardiography (EKG), Hemodynamic Scoring, and Mobile Tracking. AI architecture, distribution of manufacturers, regulatory pathways, and level of clinical testing were analyzed using data extracted from each device’s FDA approval summary documentation.
Results:
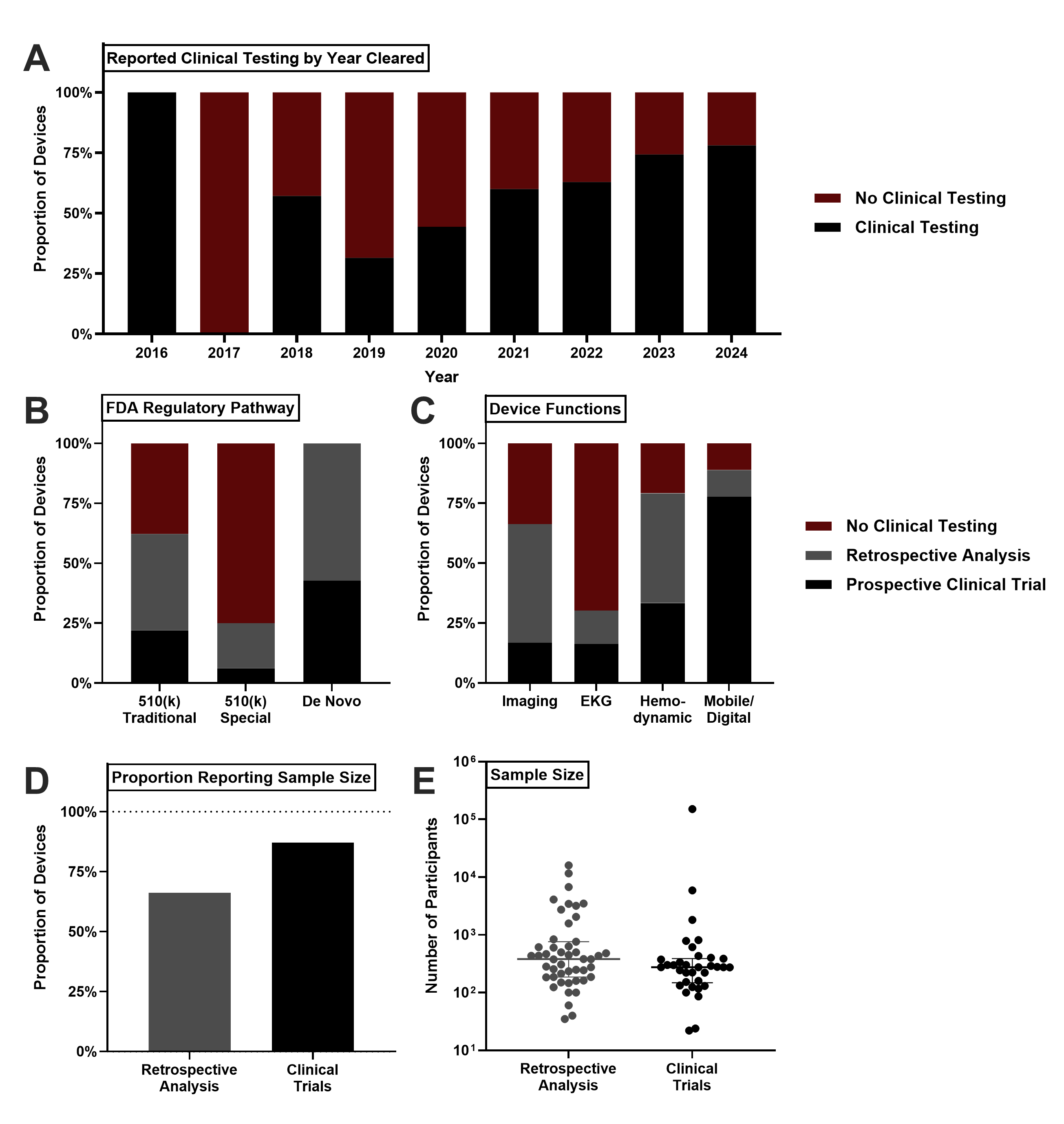
Cardiovascular AI/ML devices clearances have significantly risen, with 75.3% of devices cleared from 2020-2024(R2=0.7833). The two largest indications were Imaging (58.8%) and EKG (23.6%). AI architecture reporting from 2016-2019 was less than 50% but rapidly increased with deep learning and machine learning representing 50.0% and 34.4% of devices, respectively[BL1] [SP2] . US manufacturers produced 58.5% of devices overall, with Europe and Asia producing 17.0% and 15.4% of devices, respectively. Clinical testing varied significantly: 39.6% of devices reported no testing, 39.0% used retrospective validation, and 21.4% performed prospective trials. Notably, 69.8% of EKG devices did not report clinical testing, while 77.8% of mobile tracking devices performed prospective trials.
Conclusion:
FDA-cleared cardiovascular AI/ML devices experienced increased production, architectural capacity, and rises in clinical reporting. However, significant gaps remain in reporting. Enhanced regulatory frameworks and clinical validation are needed to ensure safety and provide clinicians with a better understanding of future technologies.
Artificial Intelligence and machine learning (AI/ML) technologies are rapidly expanding in the field of medicine, as well as cardiology. Studies within cardiovascular medicine have examined the unique capabilities, potential, and critical gaps in these devices. However, there is limited review of the types of devices that are being cleared by the U.S. Food and Drug Administration (FDA). The FDA primarily regulates new AI/ML devices, and understanding cleared devices may provide a better understanding of the landscape.
Research Question:
What are the characteristics of AI architecture, clinical indications, and regulatory pathways of devices that receive FDA clearance?
Aims:
This study aims to analyze the trends of FDA clearances of AI/ML-enabled cardiovascular devices, changes in device characteristics, and pre-clearance clinical validation of new devices.
Methods:
Data was collected from the FDA AI/ML-enabled Medical Devices Database. Of the 1,016 devices cleared, 182 cardiovascular devices were identified. Devices were divided into four clinical indications: Imaging, Electrocardiography (EKG), Hemodynamic Scoring, and Mobile Tracking. AI architecture, distribution of manufacturers, regulatory pathways, and level of clinical testing were analyzed using data extracted from each device’s FDA approval summary documentation.
Results:
Cardiovascular AI/ML devices clearances have significantly risen, with 75.3% of devices cleared from 2020-2024(R2=0.7833). The two largest indications were Imaging (58.8%) and EKG (23.6%). AI architecture reporting from 2016-2019 was less than 50% but rapidly increased with deep learning and machine learning representing 50.0% and 34.4% of devices, respectively[BL1] [SP2] . US manufacturers produced 58.5% of devices overall, with Europe and Asia producing 17.0% and 15.4% of devices, respectively. Clinical testing varied significantly: 39.6% of devices reported no testing, 39.0% used retrospective validation, and 21.4% performed prospective trials. Notably, 69.8% of EKG devices did not report clinical testing, while 77.8% of mobile tracking devices performed prospective trials.
Conclusion:
FDA-cleared cardiovascular AI/ML devices experienced increased production, architectural capacity, and rises in clinical reporting. However, significant gaps remain in reporting. Enhanced regulatory frameworks and clinical validation are needed to ensure safety and provide clinicians with a better understanding of future technologies.
More abstracts on this topic:
A Competency-Based Screening Echocardiography Curriculum Designed for Rural American Indian Community Health Representatives
Thoroughman Rose, Riley Alan, De Loizaga Sarah, Adams David, Beaton Andrea, Buonfiglio Samantha, Danforth Kristen, Masyuko Sarah, Miller Mccall, Yadava Mrinal
A Rare Case of Sequential Impella Mechanical Failures due to Infective Endocarditis VegetationsSawalski Cathryn, Seu Michelle, Darki Amir