Final ID: MP444
Once Weekly Utreglutide (GL0034), a Glucagon-like Peptide-1 Receptor Agonist Reduces Liver Fat Content along with Blood Pressure in Post-menopausal Females with Obesity: A Phase 1a/2b Study
Abstract Body (Do not enter title and authors here): Background
Utreglutide (GL0034, UTG), a novel, once weekly glucagon-like peptide-1 receptor agonist (GLP-1RA), demonstrated clinically relevant reductions of systolic and diastolic blood pressure in post-menopausal females in phase 1 trial along with improved performance in metabolic effects.
Aim
Phase 1b/2a study to evaluate the efficacy, safety, tolerability, and pharmacokinetics of utreglutide in the treatment of MAFLD.
Methods
In this randomized, double-blind, placebo-controlled parallel group multicenter study 48 participants both male and females, aged 18 to 70 years old with a body mass index (BMI) ≥30 kg/m2 and liver fat content ≥ 10% as assessed by MRI-proton density fat fraction (PDFF) were randomized (3:1) to subcutaneous UTG doses with dose titration period (2×0.4, 4×0.8, 4×1.6 mg) of 10 weeks and fixed dose (2.4 mg) period of 3 weeks; or placebo once weekly for 13 weeks. Safety, tolerability, and key efficacy along with exploratory biomarkers were assessed at baseline and week 14 follow-up visit. Biomarker analysis of stratified female participants, who were post-menopausal included body weight (BW), waist circumference (WC), systolic- and diastolic BP along with heart rate, lipid markers [triglycerides (TG), total cholesterol (TC), low density lipoprotein (LDL), Apolipoprotein B (ApoB)] leptin, MRI-PDFF, N-terminal propeptide of type III collagen (Pro-C3), urate and Leptin.
Results
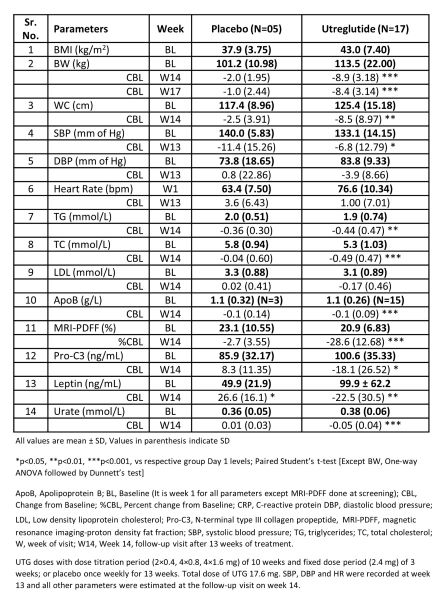
Out of 48 participants enrolled, 22 females were identified as post-menopausal, distributed as 5:17 in placebo and UTG group. This subgroup tolerated UTG well and related adverse effects were mainly gastrointestinal with nausea and vomiting. The BW reduction at Week 14 was 8.9 kg which sustained till EoS and was accompanied by 8.5 cm reduction in WC. This translated as a 6.8 mm of Hg reduction in systolic and 3.9 mm of Hg reduction in diastolic BP with no change in the heart rate. The liver fat content was reduced by 28.6 % which was supported by improvement in Pro-C3 levels by 18.1 ng/mL and lipid biomarkers. Further reductions in leptin and urate levels were also significant (Table).
Conclusions
Once weekly utreglutide dosing for thirteen weeks in post-menopausal females with obesity was well tolerated and demonstrated reductions of body weight, liver fat, systolic and diastolic blood pressure, lipids, leptin and urate.
Utreglutide (GL0034, UTG), a novel, once weekly glucagon-like peptide-1 receptor agonist (GLP-1RA), demonstrated clinically relevant reductions of systolic and diastolic blood pressure in post-menopausal females in phase 1 trial along with improved performance in metabolic effects.
Aim
Phase 1b/2a study to evaluate the efficacy, safety, tolerability, and pharmacokinetics of utreglutide in the treatment of MAFLD.
Methods
In this randomized, double-blind, placebo-controlled parallel group multicenter study 48 participants both male and females, aged 18 to 70 years old with a body mass index (BMI) ≥30 kg/m2 and liver fat content ≥ 10% as assessed by MRI-proton density fat fraction (PDFF) were randomized (3:1) to subcutaneous UTG doses with dose titration period (2×0.4, 4×0.8, 4×1.6 mg) of 10 weeks and fixed dose (2.4 mg) period of 3 weeks; or placebo once weekly for 13 weeks. Safety, tolerability, and key efficacy along with exploratory biomarkers were assessed at baseline and week 14 follow-up visit. Biomarker analysis of stratified female participants, who were post-menopausal included body weight (BW), waist circumference (WC), systolic- and diastolic BP along with heart rate, lipid markers [triglycerides (TG), total cholesterol (TC), low density lipoprotein (LDL), Apolipoprotein B (ApoB)] leptin, MRI-PDFF, N-terminal propeptide of type III collagen (Pro-C3), urate and Leptin.
Results
Out of 48 participants enrolled, 22 females were identified as post-menopausal, distributed as 5:17 in placebo and UTG group. This subgroup tolerated UTG well and related adverse effects were mainly gastrointestinal with nausea and vomiting. The BW reduction at Week 14 was 8.9 kg which sustained till EoS and was accompanied by 8.5 cm reduction in WC. This translated as a 6.8 mm of Hg reduction in systolic and 3.9 mm of Hg reduction in diastolic BP with no change in the heart rate. The liver fat content was reduced by 28.6 % which was supported by improvement in Pro-C3 levels by 18.1 ng/mL and lipid biomarkers. Further reductions in leptin and urate levels were also significant (Table).
Conclusions
Once weekly utreglutide dosing for thirteen weeks in post-menopausal females with obesity was well tolerated and demonstrated reductions of body weight, liver fat, systolic and diastolic blood pressure, lipids, leptin and urate.
More abstracts on this topic:
A Woman's Heart: Unveiling CMD as the Cause of Recurrent Syncope
Alvarez Betancourt Alejandro, Balasubramanian Suryakumar, Saladin Gustavo, Makaryus Amgad, Zeltser Roman
Accelerometer-Measured Physical Activity and Sedentary Behavior and Risks of All-Cause and Cardiovascular Disease Mortality Among Postmenopausal Cancer Survivors: The Women’s Health Accelerometry CollaborationHyde Eric, Stefanick Marcia, Skiba Meghan, Crane Tracy, Lee I-min, Lacroix Andrea, Bandoli Gretchen, Zou Jingjing, Crespo Noe, Parada Humberto, Evenson Kelly, Lamonte Michael, Nguyen Steve, Howard Annie Green