Final ID: MP1891
CPSF7-Mediated Alternative Polyadenylation of CCNE2 Enhances the Regenerative Capacity of Human iPSC-Derived Cardiomyocytes for Myocardial Infarction Therapy
Abstract Body (Do not enter title and authors here): Background: The loss of cardiac regenerative capacity after birth remains a major barrier to effective heart repair. Alternative polyadenylation (APA) has emerged as a key post-transcriptional regulatory mechanism influencing gene expression. We aimed to elucidate the role of the cleavage and polyadenylation specificity factor subunit 7 (CPSF7) in cardiomyocyte proliferation and cardiac regeneration through APA-dependent modulation of CCNE2.
Methods: Cross-species developmental analyses (mouse and human) were used to evaluate the relationship between CPSF7 and cardiomyocyte cell cycle activity. A CPSF7 conditional knockout mouse line (Cpsf7flox/flox and Myh6-Cre/Esr1, CPSF7-/-) and CPSF7-deficient human iPSC-derived cardiomyocytes (CPSF7ko-hiPSC-CMs) were generated via CRISPR/Cas9. APA regulation of CCNE2 by CPSF7 was investigated using third-generation RNA sequencing, APA site profiling, qPCR, western blotting, and immunofluorescence. The therapeutic effects of CPSF7 modulation were assessed in a mouse myocardial infarction (MI) model through both endogenous repair and exogenous hiPSC-CM transplantation, evaluating cardiomyocyte proliferation, angiogenesis, and apoptosis.
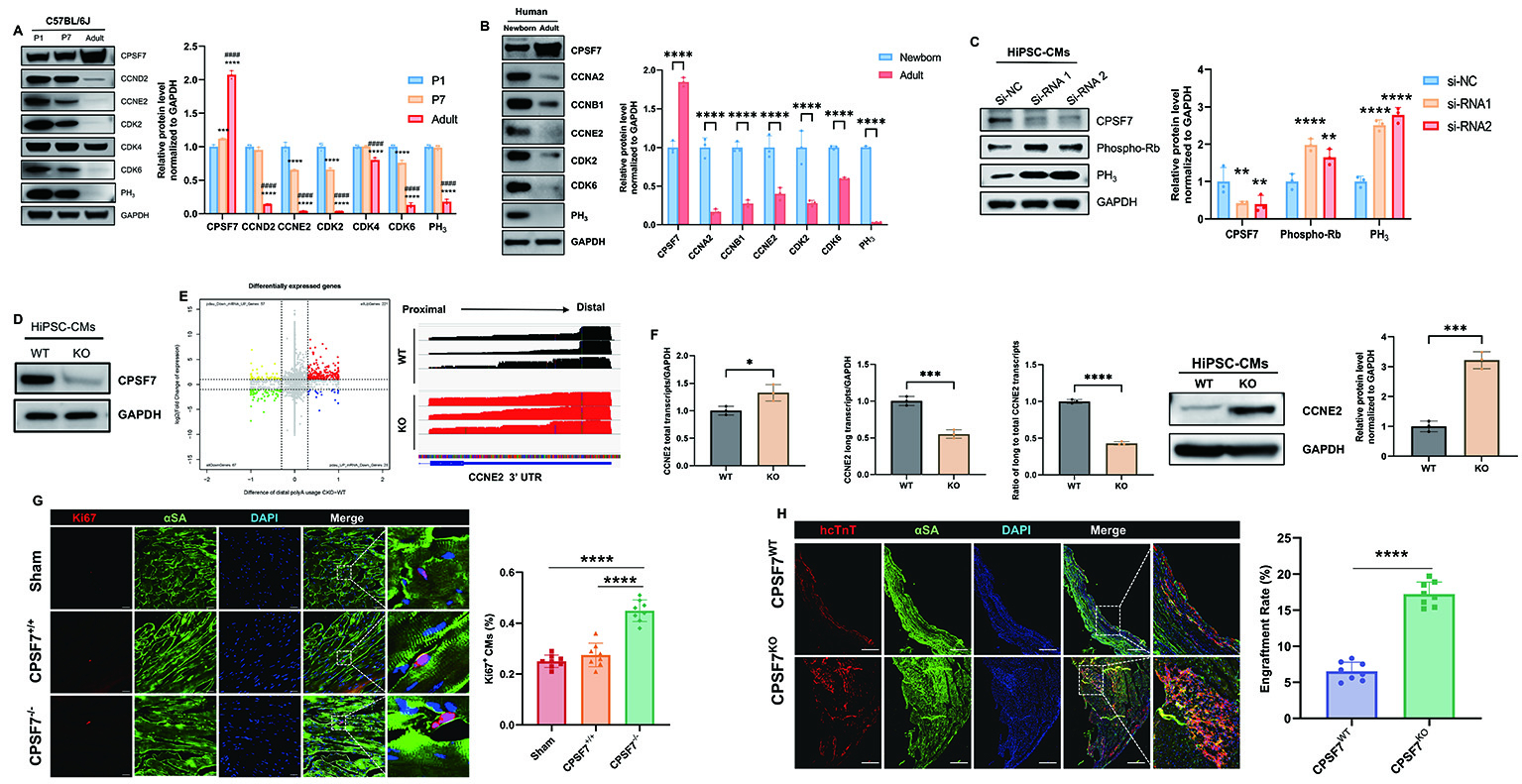
Results: Mechanistic Insights: CPSF7 expression was elevated during postnatal cardiac maturation and was found to negatively regulate the cell cycle activation of cardiomyocytes (Panel A-C). Deletion of CPSF7 resulted in 3′ untranslated region (3′UTR) shortening of CCNE2 transcripts, leading to enhanced protein expression and increased cardiomyocyte proliferation (Panel D-F); Endogenous Repair: CPSF7-/- mice exhibited significantly improved post-MI cardiac function, reduced infarct size, and increased cardiomyocytes proliferation compared to wild-type controls (Panel G). Apoptotic cardiomyocytes were reduced by 62%; Exogenous Cell Therapy: Transplantation of CPSF7ko-hiPSC-CMs into infarcted hearts led to a 3-fold increase in cell retention compared to wild-type hiPSC-CMs (Panel H). Treated mice demonstrated a 24% improvement in ejection fraction and enhanced myocardial regeneration relative to control groups.
Conclusion: CPSF7 represses CCNE2 expression via APA-dependent 3′UTR lengthening, serving as a key brake on cardiomyocyte proliferation. Targeted CPSF7 inhibition activates endogenous cardiac repair mechanisms and enhances the therapeutic efficacy of transplanted hiPSC-derived cardiomyocytes.
Methods: Cross-species developmental analyses (mouse and human) were used to evaluate the relationship between CPSF7 and cardiomyocyte cell cycle activity. A CPSF7 conditional knockout mouse line (Cpsf7flox/flox and Myh6-Cre/Esr1, CPSF7-/-) and CPSF7-deficient human iPSC-derived cardiomyocytes (CPSF7ko-hiPSC-CMs) were generated via CRISPR/Cas9. APA regulation of CCNE2 by CPSF7 was investigated using third-generation RNA sequencing, APA site profiling, qPCR, western blotting, and immunofluorescence. The therapeutic effects of CPSF7 modulation were assessed in a mouse myocardial infarction (MI) model through both endogenous repair and exogenous hiPSC-CM transplantation, evaluating cardiomyocyte proliferation, angiogenesis, and apoptosis.
Results: Mechanistic Insights: CPSF7 expression was elevated during postnatal cardiac maturation and was found to negatively regulate the cell cycle activation of cardiomyocytes (Panel A-C). Deletion of CPSF7 resulted in 3′ untranslated region (3′UTR) shortening of CCNE2 transcripts, leading to enhanced protein expression and increased cardiomyocyte proliferation (Panel D-F); Endogenous Repair: CPSF7-/- mice exhibited significantly improved post-MI cardiac function, reduced infarct size, and increased cardiomyocytes proliferation compared to wild-type controls (Panel G). Apoptotic cardiomyocytes were reduced by 62%; Exogenous Cell Therapy: Transplantation of CPSF7ko-hiPSC-CMs into infarcted hearts led to a 3-fold increase in cell retention compared to wild-type hiPSC-CMs (Panel H). Treated mice demonstrated a 24% improvement in ejection fraction and enhanced myocardial regeneration relative to control groups.
Conclusion: CPSF7 represses CCNE2 expression via APA-dependent 3′UTR lengthening, serving as a key brake on cardiomyocyte proliferation. Targeted CPSF7 inhibition activates endogenous cardiac repair mechanisms and enhances the therapeutic efficacy of transplanted hiPSC-derived cardiomyocytes.
More abstracts on this topic:
An Analysis of Ischemic Heart Disease Mortality Trends Attributable to High Body Mass Index in High-Income Countries: Global Burden of Disease Study from 1990-2021
Shaar Abdalkader, Bhat Rakshita Ramesh, Khan Wajeeh, Khan Wajeeha
A Perfect Storm: Simultaneous Pulmonary Embolism, STEMI, and Stroke via Paradoxical Embolism in a Hospitalized Patient on DVT ProphylaxisKhan Abdul Allam, Thukral Jatin, Elgabry Ibrahim, Lamp Garron