Final ID: MP1855
Endothelial cell specific senescent cell clearance alleviates metabolic dysfunction in obese mice
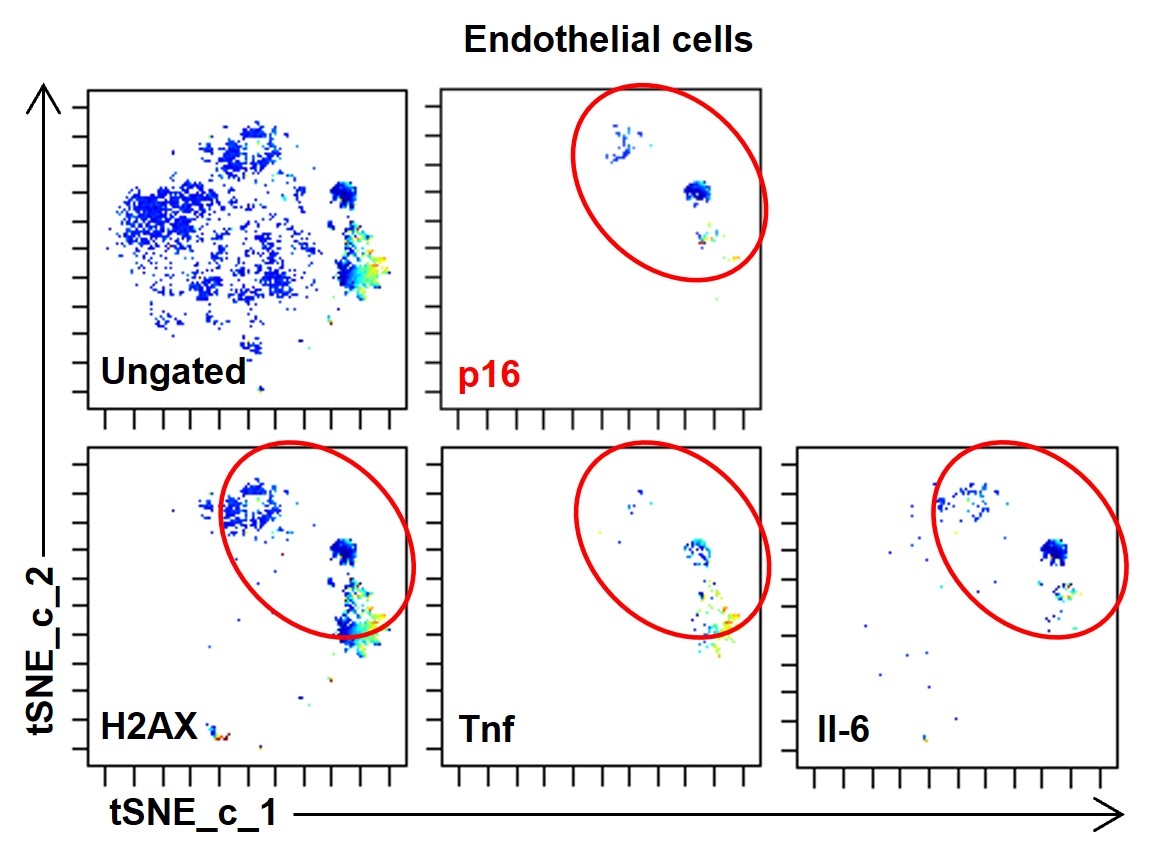
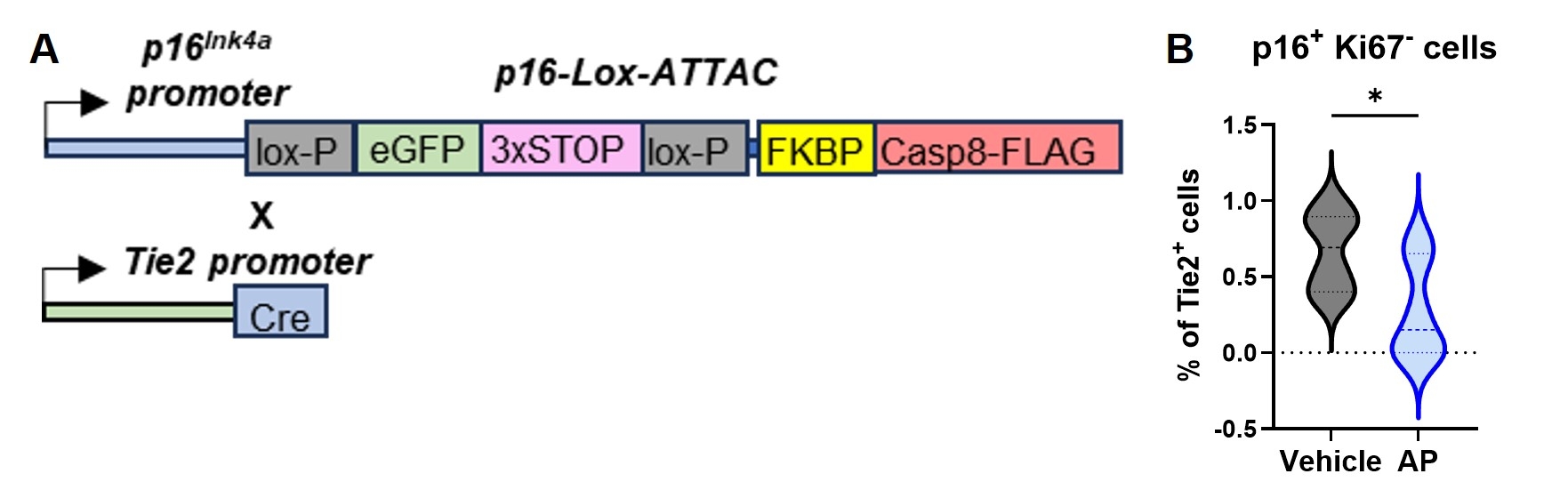
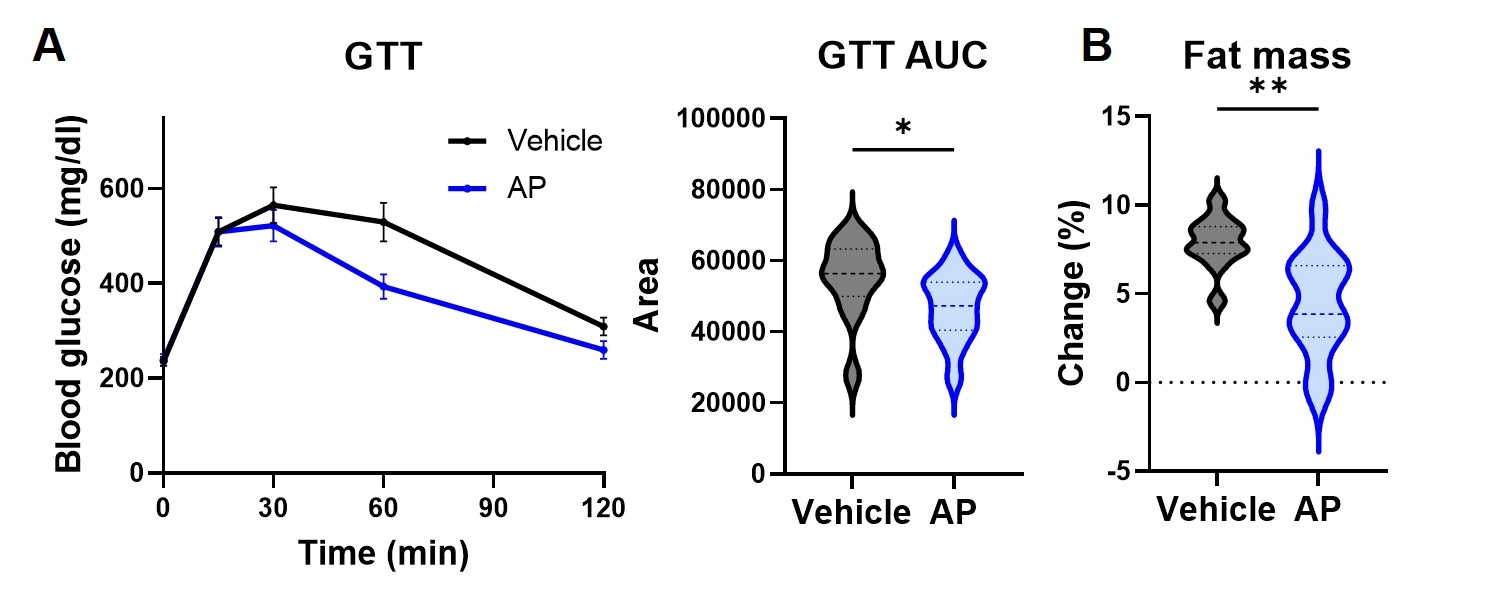
Abstract Body (Do not enter title and authors here): Aging is the leading risk factor of chronic diseases including cardiovascular diseases and metabolic diseases. Emerging evidence suggests cellular senescence, one of the pillars of aging, plays a causal role in development of these diseases. While we previously reported that senescent cell elimination delays or prevents progression of several aging phenotypes in aged mice, the responsible types of senescent cells contributing to diseases in vivo are not yet fully elucidated. We made high fat diet-induced obesity model (60% fat diet, HFD) and examine the cell type of senescent cells. Single cell mass cytometry analysis using adipose tissue revealed that senescent endothelial cells expressed the DNA damage marker γH2AX and inflammatory factors such as TNFα and IL-6 (Figure 1). To test the hypothesis that senescent endothelial cells are a major contributor to chronic diseases, we developed Tie2-Cre; p16Ink4a-LOX-ATTAC mice, which allows specific elimination of p16Ink4a positive senescent endothelial cells upon administration of AP20187 (which only affects Tie2-expressing p16Ink4a positive cells carrying the conditionally-expressed ATTAC construct) (Figure 2). We found that targeted removal of these cells alleviated HFD-induced metabolic dysfunction with decrease of inflammatory cytokines (Figure 3). In contrast, transplanting senescent endothelial cells into lean mice caused adipose tissue inflammation and metabolic dysfunction. We found the inflammatory factors from senescent endothelial cells induced inflammation in preadipocytes and expand inflammation and targeting senescent endothelial cells may cut the expanding inflammation. Consistent with these findings, natural flavonoid fisetin, which we previously identified that can induce apoptosis selectively to senescent endothelial cells among other senescent cell types, reduced senescent cell amount in adipose tissue and improved glucose metabolism. Moreover, we confirmed the effect of fisetin against senescent endothelial cells in freshly isolated human omental adipose tissue from obese individuals. The fisetin-treated explants exhibited a significant reduction in both SA-β-gal-positive adipocytes and endothelial cells, as well as fewer p16-positive cells, indicating that fisetin induced apoptosis in senescent endothelial cells. Collectively, these data suggest that elimination of p16Ink4a positive senescent endothelial cells may be a viable therapeutic strategy for alleviation of metabolic diseases.
More abstracts on this topic:
Activation of TRPA1 with allyl isothiocyanate prevents age-related cardiac diastolic dysfunction
Qian Chunqi, Fernandez Zachary, Wang Donna, Ma Shuangtao
4D Flow MRI Evaluation of Cardiovascular Risk-Related Alterations in Heart-Brain Hemodynamics in Cognitively Healthy Aging AdultsNajafi Anahita, Rogalski Emily, Jarvis Kelly, Richter Adam, Lytchakov Anna, Benson Theresa, Jin Ning, Davids Rachel, Schnell Susanne, Ragin Ann, Weintraub Sandra