Final ID: Sa2046
Improvements in Cardiometabolic Risk Factors by Body Weight Reduction with Tirzepatide in Adults with Obesity and Prediabetes: A Post Hoc Analysis of the 3-year SURMOUNT-1 study
Abstract Body (Do not enter title and authors here): Background: In the 3-year SURMOUNT-1 study in people with obesity and prediabetes, tirzepatide treatment was associated with significant and consistent body weight reduction, lower risk of progression to type 2 diabetes, and significant improvement in cardiometabolic risk factors compared with placebo. This post hoc analysis assessed changes in cardiometabolic risk factors by body weight reduction thresholds with tirzepatide.
Methods: Participants (N=458) randomized to receive tirzepatide (pooled 5/10/15 mg doses) and with an on-treatment body weight measurement at Week 176 were included in the analysis. The proportion of participants achieving body weight reduction thresholds (<5%, 5 to <10%, 10 to <15%, 15 to <20%, 20 to <25%, 25 to <30%, 30 to <35%, and ≥35%) at Week 176 and change from baseline at Week 176 in cardiometabolic risk factors, including blood pressure, lipid profile, and glycemic parameters, by body weight reduction thresholds were assessed.
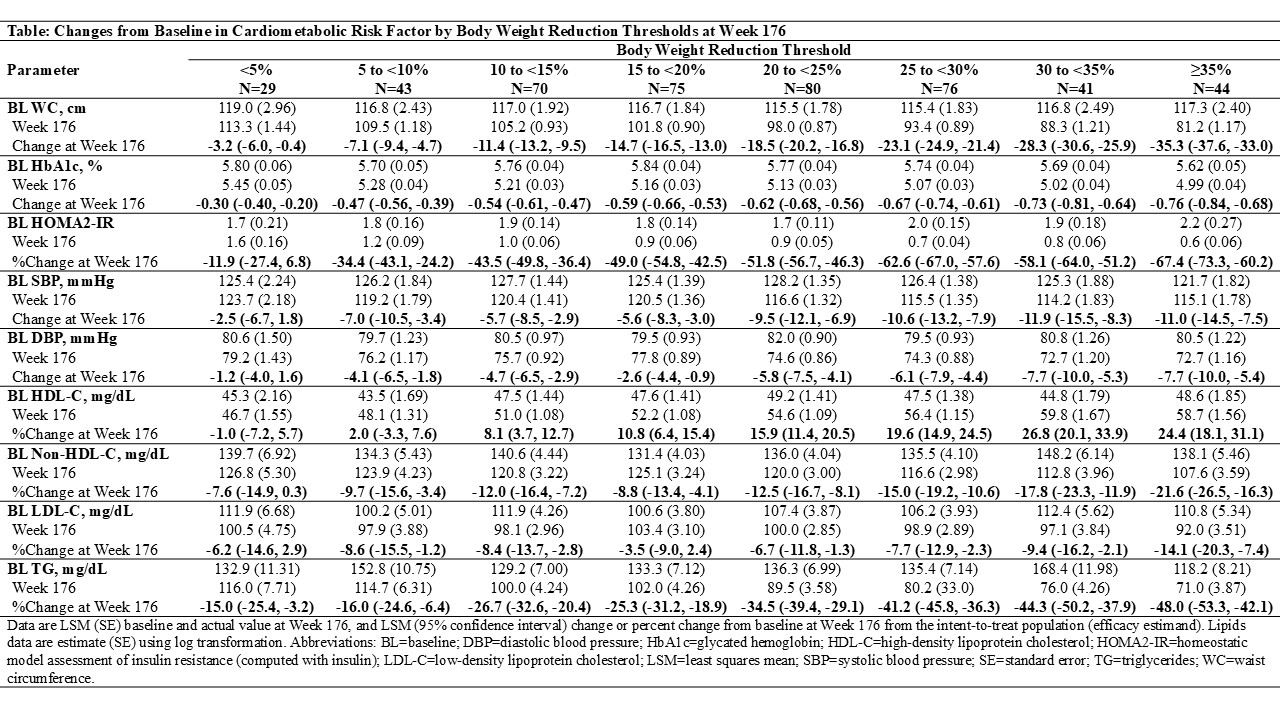
Results: At baseline, mean waist circumference was 116.5 cm, systolic blood pressure: 126.1 mmHg, diastolic blood pressure: 80.4 mmHg, triglycerides: 153.1 mg/dL, HDL-C: 48.6 mg/dL, non-HDL-C: 141.6 mg/dL, LDL-C: 111.8 mg/dL, and HOMA-2-IR (computed with insulin): 2.1. Improvements in waist circumference (3.2-35.3 cm reduction), HbA1c (0.30-0.76% reduction) and triglycerides (15-48% reduction) were observed among all body weight reduction subgroups (Table). HOMA2-IR, blood pressure and HDL-C, non-HDL-C and LDL-C also improved among participants who lost ≥5% of body weight. Overall, greater improvements in cardiometabolic risk factors were generally associated with greater body weight reduction.
Conclusions: In this post hoc analysis of the 3-year SURMOUNT-1 study, tirzepatide treatment was associated with improved cardiometabolic risk factors, that were generally greater in magnitude with higher degrees of body weight reduction among people with obesity and prediabetes.
Methods: Participants (N=458) randomized to receive tirzepatide (pooled 5/10/15 mg doses) and with an on-treatment body weight measurement at Week 176 were included in the analysis. The proportion of participants achieving body weight reduction thresholds (<5%, 5 to <10%, 10 to <15%, 15 to <20%, 20 to <25%, 25 to <30%, 30 to <35%, and ≥35%) at Week 176 and change from baseline at Week 176 in cardiometabolic risk factors, including blood pressure, lipid profile, and glycemic parameters, by body weight reduction thresholds were assessed.
Results: At baseline, mean waist circumference was 116.5 cm, systolic blood pressure: 126.1 mmHg, diastolic blood pressure: 80.4 mmHg, triglycerides: 153.1 mg/dL, HDL-C: 48.6 mg/dL, non-HDL-C: 141.6 mg/dL, LDL-C: 111.8 mg/dL, and HOMA-2-IR (computed with insulin): 2.1. Improvements in waist circumference (3.2-35.3 cm reduction), HbA1c (0.30-0.76% reduction) and triglycerides (15-48% reduction) were observed among all body weight reduction subgroups (Table). HOMA2-IR, blood pressure and HDL-C, non-HDL-C and LDL-C also improved among participants who lost ≥5% of body weight. Overall, greater improvements in cardiometabolic risk factors were generally associated with greater body weight reduction.
Conclusions: In this post hoc analysis of the 3-year SURMOUNT-1 study, tirzepatide treatment was associated with improved cardiometabolic risk factors, that were generally greater in magnitude with higher degrees of body weight reduction among people with obesity and prediabetes.
More abstracts on this topic:
Adiposity and Cardiac Function in South Asian Americans: Findings from the MASALA Study
Kanaya Alka, Nelson Lauren, Running Allison, Lin Feng, Kandula Namratha, Gadgil Meghana, Win Sithu, Shah Sanjiv
Comparison of Dietary Macronutrient Interventions for Weight and Cardiovascular Risk Factor Reduction: A Systematic Review and Network Meta-analysis of Randomized Controlled TrialAli Eman, Hall Michael And Jo Alice, Latif Fakhar, Ali Kumail Mustafa, Perswani Prinka, Janjua Hamza, Ansari Yusra, Vipparthy Sharath, Ali Farman, Siddiqi Tariq Jamal