Final ID: MDP1561
Inflammatory biomarkers in people treated with tirzepatide living with overweight or obesity, without and with T2D: a post-hoc analysis from SURMOUNT-1 and SURMOUNT-2
Abstract Body (Do not enter title and authors here): Background: Tirzepatide (TZP) is a once weekly GIP and GLP-1 receptor agonist approved for the treatment of type 2 diabetes (T2D) and obesity. This post hoc analysis examined biomarkers of inflammation in people living with obesity, or overweight, without and with T2D, from SURMOUNT-1 and SURMOUNT-2. Furthermore, we evaluated the contribution of weight reduction- associated and - unassociated effects on biomarkers of inflammation.
Methods: A total of 700 participants were randomly selected from SURMOUNT-1 and SURMOUNT-2 (100 participants from each treatment arm: placebo, 5, 10, and 15 mg in SURMOUNT-1 and placebo, 10 and 15 mg in SURMOUNT-2). The association of treatment with change from baseline in the inflammation biomarkers interleukin-6 (IL-6) and high sensitivity C-reactive protein (hsCRP) at 24 and 72 weeks was assessed along with the estimated percentages of the association attributable to weight loss through a mediation analysis.
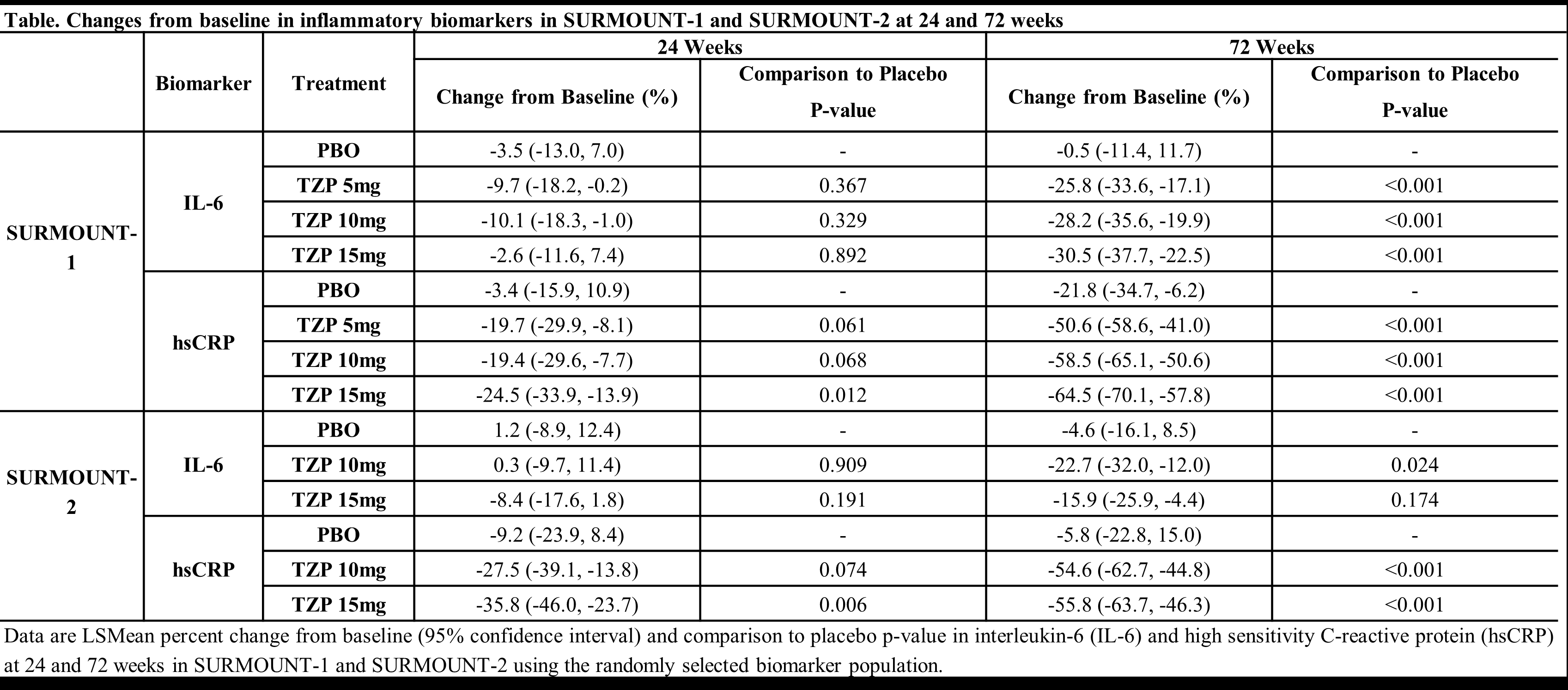
Results: In SURMOUNT-1, following 72 weeks of treatment with TZP without T2D, TZP was associated with significantly decreased IL-6 (-26% to -31%) and hsCRP (-51% to -65%), compared to placebo in all dose groups. In SURMOUNT-2, following 72 weeks of treatment with TZP in participants with T2D, changes of -16% to -23% in IL-6 and -55% to -56% in hsCRP were observed (significance seen for all groups except for TZP 15mg on IL-6). At 24 weeks, only 18% and 31% of hsCRP changes were associated with weight reduction in SURMOUNT-1 and -2, respectively. In SURMOUNT-1, 77% of IL-6 changes and 87% of hsCRP changes at 72 weeks were associated with weight reduction. In SURMOUNT-2, 78% of IL-6 changes and 57% of hsCRP changes at 72 weeks were associated with body weight reduction.
Conclusions: In this post hoc analysis of SURMOUNT-1 and -2, early changes in hsCRP (week 24) were weight reduction-unassociated, while at week 72, changes in the inflammation biomarkers IL-6 and hsCRP observed in TZP-treated participants were mainly weight reduction-associated. The relative contribution of weight reduction-dependent effects was more prominent in participants without T2D, compared to those with T2D. Collectively, these data suggest TZP was associated with reduced inflammation in people with overweight/obesity and/or T2D.
Methods: A total of 700 participants were randomly selected from SURMOUNT-1 and SURMOUNT-2 (100 participants from each treatment arm: placebo, 5, 10, and 15 mg in SURMOUNT-1 and placebo, 10 and 15 mg in SURMOUNT-2). The association of treatment with change from baseline in the inflammation biomarkers interleukin-6 (IL-6) and high sensitivity C-reactive protein (hsCRP) at 24 and 72 weeks was assessed along with the estimated percentages of the association attributable to weight loss through a mediation analysis.
Results: In SURMOUNT-1, following 72 weeks of treatment with TZP without T2D, TZP was associated with significantly decreased IL-6 (-26% to -31%) and hsCRP (-51% to -65%), compared to placebo in all dose groups. In SURMOUNT-2, following 72 weeks of treatment with TZP in participants with T2D, changes of -16% to -23% in IL-6 and -55% to -56% in hsCRP were observed (significance seen for all groups except for TZP 15mg on IL-6). At 24 weeks, only 18% and 31% of hsCRP changes were associated with weight reduction in SURMOUNT-1 and -2, respectively. In SURMOUNT-1, 77% of IL-6 changes and 87% of hsCRP changes at 72 weeks were associated with weight reduction. In SURMOUNT-2, 78% of IL-6 changes and 57% of hsCRP changes at 72 weeks were associated with body weight reduction.
Conclusions: In this post hoc analysis of SURMOUNT-1 and -2, early changes in hsCRP (week 24) were weight reduction-unassociated, while at week 72, changes in the inflammation biomarkers IL-6 and hsCRP observed in TZP-treated participants were mainly weight reduction-associated. The relative contribution of weight reduction-dependent effects was more prominent in participants without T2D, compared to those with T2D. Collectively, these data suggest TZP was associated with reduced inflammation in people with overweight/obesity and/or T2D.
More abstracts on this topic:
Aging Heart Failure with Preserved Ejection Fraction is Mediated by Noncoding RNAs
Chakraborty Sankalpa, Dickerson Bryce, Bounds Curren, Lemus Sophia, Hickman Caleb, Rajagopalan Viswanathan
A multi-proteomic Risk Score Predicts Adverse Cardiovascular Outcomes in Patients with Angina and Non-obstructive Coronary Artery DiseaseHuang Jingwen, Lodhi Rafia, Lodhi Saleha, Eldaidamouni Ahmed, Hritani Wesam, Hasan Muhammet, Haroun Nisreen, Quyyumi Arshed, Mehta Puja, Leon Ana, Ko Yi-an, Yang Huiying, Medina-inojosa Jose, Ahmed Taha, Harris Kristen, Alkhoder Ayman, Al Kasem Mahmoud