Final ID: MP1484
Lipid lowering effects of inclisiran in statin- or PCSK9i monoclonal antibody-intolerant patients
Inclisiran is a small-interfering RNA therapy that lowered plasma LDL-C levels by ~50% at 90 days in placebo-controlled trials. The LDL-C lowering effects of inclisiran in patients with prior adverse effects (AEs) to statins or PCSK9 inhibiting (PCSK9i) monoclonal antibody therapies (mAbs), who may have limited therapeutic options and experience suboptimal LDL-C control, is not well-known.
Research Question
What are the LDL-C lowering effects of inclisiran in patients who have a history of AEs to statins and/or PCSK9i mAbs?
Methods
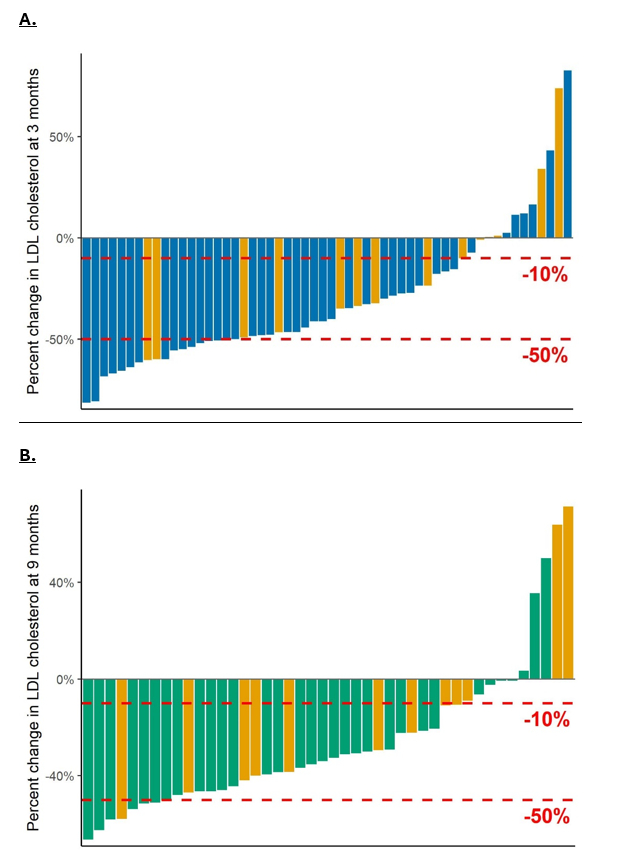
At a tertiary US preventive cardiology clinic, we retrospectively evaluated electronic health records of patients > 18 years of age who received > 1 dose of inclisiran between 1/1/2022 and 2/29/2024. LDL-C was measured at 3 and 9 months (mos). Median (IQR) values were reported for LDL-C reductions because data were not normally distributed.
Results
Of 62 patients, 56 had available LDL-C data at 3 mos, 57 received a second dose, and 44 had available LDL-C data at 9 mos. The mean age was 67.3 y (SD 10.9), 60.3% were female, 44.4% were secondary prevention, 28.6% had at least possible FH by Dutch Lipid Clinic Network criteria (> 3 points), 33.3% were receiving statins at baseline, and 41.3% were receiving ezetimibe at baseline. A total of 55/62 (89%) had a history of prior AEs from statins at baseline, and 17/62 (27.4%) had a history of prior PCSK9i-mAb AEs at baseline. The mean LDL-C at baseline was 137.2 mg/dL (SD 62.9). Median lipoprotein (a) was 27.5 mg/dL (IQR 10.5 to 86.5). At 3 mos, overall median reduction was 40.7% (IQR 15.7 to 51.7). A total of 11/56 (19.6%) patients had <10% LDL-C reduction. Median LDL-C reductions were 40.7% (IQR 9.3 to 50.7) in patients with statin AEs, and 23.6% (IQR 0.5 to 46.7) in patients with PCSK9i mAb AEs. Upon excluding patients with prior PCSK9i mAb AEs, the median 3-mo reduction was 46.4% (IQR 25.4 to 54.4). At 9 mos, the median overall LDL-C reduction was 33.2% (95% CI 10.9 to 46.4).
Conclusion
In a retrospective cohort of patients mostly with a history of AEs from statins and/or PCSK9i mAbs, inclisiran use was associated with lower percent LDL-C reductions at 3 and 9 mos than reported in placebo-controlled trials, driven by attenuated LDL-C reductions in patients with prior PCSK9i mAb AEs. Exclusion of patients with prior PCSK9i mAb use with AEs led to similar overall LDL-C lowering as in trials. Further investigation is warranted.
More abstracts on this topic:
Ono Yoshiyasu, Ikeda Shota, Shinohara Keisuke, Matsumoto Sho, Yoshida Daisuke, Nakashima Ryosuke, Nakashima Hiroka, Miyamoto Ryohei, Abe Kohtaro
A transformative LDL cholesterol–lowering in vivo CRISPR gene editing medicine that functionally upregulates LDLR in mice and non-human primatesNewmark Judith, Raghav Jimit, Jaskolka Michael, Diner Benjamin, Soman Vikram, Jinadasa Tushare, Apte Ameya, Wu Meng, Bottega Steve, Thakkar Mansi, Agosto Luis, Wrighton Paul, Majithia Deep, Jambard Shreya, Jansson-fritzberg Linnea, Jones Mark, Fletcher Jillian, Weiss Mckenzie, Kaye Emily, Steward Briana, Bochicchio James, Pietrasiewicz Stephen, Iovino Salvatore, Marco Rubio Eugenio, Trong Phan Huu, Chander Nisha, Kazemian Mohammadreza, Lam Kieu, Reid Steve, Dinsmore Michael, Teslovich Tanya, Xie Jenny, Gupta Anshul, Amin Parth, Burkly Linda, Thompson Morgan, Rizal Salu, Bilodeau Maxime, Dong Ruhong, Zhen Wei