Final ID: Su3146
Prevalence of Familial Hypercholesteremia (FH) Among Participants in the ACCELERATE Trial: Implications for Opportunistic FH Screening and Prognostication
Abstract Body (Do not enter title and authors here): Background: Familial hypercholesteremia (FH) leads to elevated low-density lipoprotein cholesterol (LDL-C) and atherosclerotic cardiovascular disease (ASCVD). Although treatable, FH is underdiagnosed. Lipid lowering therapy may mask diagnostic pretreatment LDL-C levels. Participants of ASCVD trials may be enriched for FH, so ASCVD trial enrollment may be a unique contact point to opportunistically diagnose FH.
Hypothesis: The population of the ACCELERATE trial of evacetrapib and ASCVD outcomes is enriched for FH.
Methods: ACCELERATE is a phase 3 cardiovascular outcomes trial which randomized 12,092 patients with high-risk vascular disease to receive evacetrapib or placebo. FH was not reported. Using participant-level data, we estimated pretreatment LDL-c using validated corrections based on type and dose of statin therapy. We defined severe hypercholesterolemia as pretreatment LDL-C ≥ 190 mg/dl and FH as severe hypercholesterolemia with total cholesterol > 290 mg/dL in a first or second degree relative, consistent with Simon Broome register criteria. We compared trial prevalence to general prevalence (severe hypercholesterolemia ~7%, FH ~0.4%). We evaluated the adjusted association of severe hypercholesterolemia with the primary trial endpoint of ASCVD events using multivariable Cox proportional hazards regression.
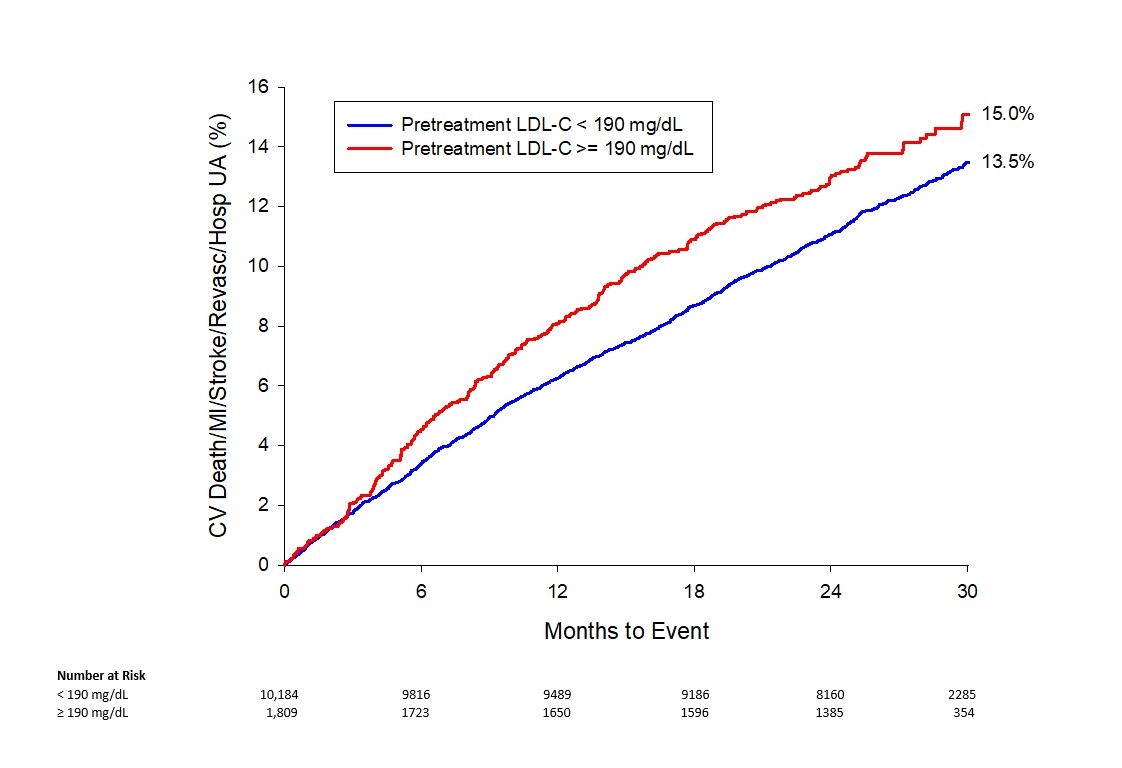
Results: Data were available for 11,993 participants (99%). The prevalence of severe hypercholesteremia was 15% (1809/11993). The prevalence of FH was 2.1% (255/11993). Pretreatment LDL-C ≥ 190 mg/dL, as compared with pretreatment LDL-C < 190 mg/dL, was significantly associated with a higher incidence of the primary ASCVD trial endpoint (15% vs 13.5% respectively, adjusted hazard ratio 1.19; 95% CI 1.03-1.38, P=0.021; Figure).
Conclusion: In a participant-level analysis of a rigorous, independently adjudicated ASCVD outcomes trial, severe hypercholesterolemia and FH were more prevalent in the trial population than the general population based on pretreatment LDL-C calculation. Severe hypercholesterolemia was significantly associated with higher ASCVD incidence. ASCVD trial enrollment may be a novel high-yield contact point for index FH case identification using simple pretreatment LDL-C calculation.
Hypothesis: The population of the ACCELERATE trial of evacetrapib and ASCVD outcomes is enriched for FH.
Methods: ACCELERATE is a phase 3 cardiovascular outcomes trial which randomized 12,092 patients with high-risk vascular disease to receive evacetrapib or placebo. FH was not reported. Using participant-level data, we estimated pretreatment LDL-c using validated corrections based on type and dose of statin therapy. We defined severe hypercholesterolemia as pretreatment LDL-C ≥ 190 mg/dl and FH as severe hypercholesterolemia with total cholesterol > 290 mg/dL in a first or second degree relative, consistent with Simon Broome register criteria. We compared trial prevalence to general prevalence (severe hypercholesterolemia ~7%, FH ~0.4%). We evaluated the adjusted association of severe hypercholesterolemia with the primary trial endpoint of ASCVD events using multivariable Cox proportional hazards regression.
Results: Data were available for 11,993 participants (99%). The prevalence of severe hypercholesteremia was 15% (1809/11993). The prevalence of FH was 2.1% (255/11993). Pretreatment LDL-C ≥ 190 mg/dL, as compared with pretreatment LDL-C < 190 mg/dL, was significantly associated with a higher incidence of the primary ASCVD trial endpoint (15% vs 13.5% respectively, adjusted hazard ratio 1.19; 95% CI 1.03-1.38, P=0.021; Figure).
Conclusion: In a participant-level analysis of a rigorous, independently adjudicated ASCVD outcomes trial, severe hypercholesterolemia and FH were more prevalent in the trial population than the general population based on pretreatment LDL-C calculation. Severe hypercholesterolemia was significantly associated with higher ASCVD incidence. ASCVD trial enrollment may be a novel high-yield contact point for index FH case identification using simple pretreatment LDL-C calculation.
More abstracts on this topic:
Combining metabolomic profiling with exome sequencing in the UK Biobank to predict cardiac outcomes and interpret variants of uncertain significance in familial hypercholesterolemia
Jostins-dean Luke, Schut Kirsten, Kerminen Sini, Wurtz Peter, Barrett Jeffrey
A Novel Thrombolytic with Anti-inflammatory Properties (JX10) Improves Neurological Outcomes in Acute Lacunar Infarct up to 12 hours After OnsetChen Edmond, Niizuma Kuniyasu, Nitika Fnu, Hasumi Keiji, Tominaga Teiji, Nishimura Naoko, Zhang Shenglin