Final ID: Su2138
Efficacy of Olpasiran by Apolipoprotein(a) Isoform Size: Insights from the OCEAN(a)-DOSE Trial
Abstract Body (Do not enter title and authors here):
Background: Lipoprotein(a) [Lp(a)] concentration is predominantly determined by genetics, with an inverse correlation between concentration and the number of Kringle IV-2 (KIV-2) repeats carried on apolipoprotein(a) [apo(a)]. The relationship between apo(a) isoform size and response to lowering of Lp(a) with olpasiran through RNA interference of apo(a) expression is not known.
Hypothesis: In patients with elevated Lp(a), the efficacy of olpasiran is independent of KIV-2 repeat number.
Methods: OCEAN(a)-DOSE was a randomized, placebo-controlled, phase 2 trial that evaluated 4 active doses of olpasiran (10 mg Q12W, 75 mg Q12W, 225 mg Q12W, 225 mg Q24W) in patients with atherosclerotic cardiovascular disease (ASCVD) and Lp(a) >150 nmol/L. With a gel electrophoresis and immunoblotting system (Bio-Rad Submarine), the number of KIV-2 repeats was determined, as well as the relative expression of each patient’s apo(a) isoform. KIV-2 repeats were modeled as a continuous and categorical variable (<17, 17-19, 20-22, >22). Lp(a) concentration was measured with a molarity-based assay (Roche). The placebo-adjusted least-square means (LSM) percent change in Lp(a) from baseline to week 36 with olpasiran was examined as a function of number of KIV-2 repeats on the dominant isoform.
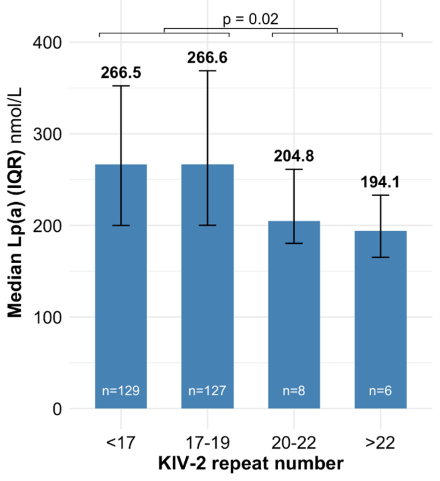
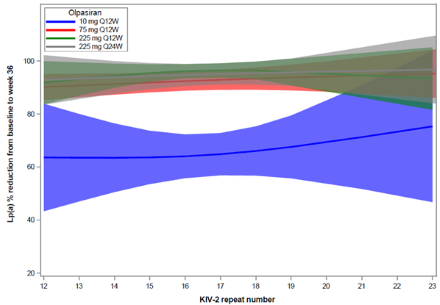
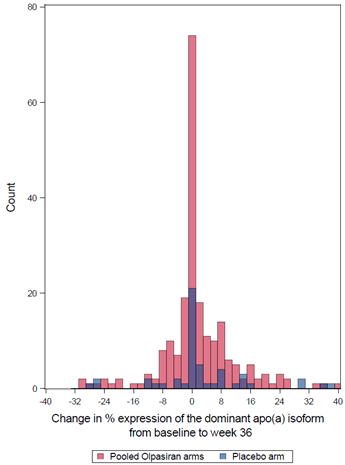
Results: A total of 270 patients had apo(a) isoform and Lp(a) available at baseline and at week 36. At baseline, the median Lp(a) [IQR] concentration was 260.2 [197.9–358.5] nmol/L. Lp(a) concentration tended to be higher among those with a lower number of KIV-2 repeats (p=0.02, Panel A). At week 36, the placebo-adjusted LSM percent change from baseline in Lp(a) with olpasiran was consistent irrespective of baseline KIV-2 repeat number (Pinteraction=0.66; Panel B). No significant change was observed over time in the percent expression of the dominant apo(a) isoform with olpasiran compared to placebo (mean change from baseline to week 36 [±SD]: +1.1 [±9.8] % with olpasiran and +2.1 [±12.8] % with placebo, p = 0.65; Panel C).
Conclusion: In patients with ASCVD and elevated Lp(a), olpasiran reduces Lp(a) irrespective of apo(a) isoform size and appears to affect both isoforms equally.
Background: Lipoprotein(a) [Lp(a)] concentration is predominantly determined by genetics, with an inverse correlation between concentration and the number of Kringle IV-2 (KIV-2) repeats carried on apolipoprotein(a) [apo(a)]. The relationship between apo(a) isoform size and response to lowering of Lp(a) with olpasiran through RNA interference of apo(a) expression is not known.
Hypothesis: In patients with elevated Lp(a), the efficacy of olpasiran is independent of KIV-2 repeat number.
Methods: OCEAN(a)-DOSE was a randomized, placebo-controlled, phase 2 trial that evaluated 4 active doses of olpasiran (10 mg Q12W, 75 mg Q12W, 225 mg Q12W, 225 mg Q24W) in patients with atherosclerotic cardiovascular disease (ASCVD) and Lp(a) >150 nmol/L. With a gel electrophoresis and immunoblotting system (Bio-Rad Submarine), the number of KIV-2 repeats was determined, as well as the relative expression of each patient’s apo(a) isoform. KIV-2 repeats were modeled as a continuous and categorical variable (<17, 17-19, 20-22, >22). Lp(a) concentration was measured with a molarity-based assay (Roche). The placebo-adjusted least-square means (LSM) percent change in Lp(a) from baseline to week 36 with olpasiran was examined as a function of number of KIV-2 repeats on the dominant isoform.
Results: A total of 270 patients had apo(a) isoform and Lp(a) available at baseline and at week 36. At baseline, the median Lp(a) [IQR] concentration was 260.2 [197.9–358.5] nmol/L. Lp(a) concentration tended to be higher among those with a lower number of KIV-2 repeats (p=0.02, Panel A). At week 36, the placebo-adjusted LSM percent change from baseline in Lp(a) with olpasiran was consistent irrespective of baseline KIV-2 repeat number (Pinteraction=0.66; Panel B). No significant change was observed over time in the percent expression of the dominant apo(a) isoform with olpasiran compared to placebo (mean change from baseline to week 36 [±SD]: +1.1 [±9.8] % with olpasiran and +2.1 [±12.8] % with placebo, p = 0.65; Panel C).
Conclusion: In patients with ASCVD and elevated Lp(a), olpasiran reduces Lp(a) irrespective of apo(a) isoform size and appears to affect both isoforms equally.
More abstracts on this topic:
2-Deoxyuridine Associates with Recurrent Coronary Events
Pistritu Dan, Castano David, Liehn Elisa, Koh Cho Yeow, Gerszten Robert, Singaraja Roshni, Chan Mark, Shah Svati
A Novel Tool for Evaluating Endothelial Function: Plethysmographic Flow-mediated Vasodilation (pFMD)Kishimoto Shinji, Itarashiki Tomomasa, Higashi Yukihito, Maruhashi Tatsuya, Kajikawa Masato, Mizobuchi Aya, Harada Takahiro, Yamaji Takayuki, Nakano Yukiko, Mohamad Yusoff Farina, Yada Tomohiko