Final ID: MP493
EVOLocumab Very Early after Myocardial Infarction (EVOLVE-MI): A Pragmatic Randomized Multicenter International Trial - Design and Baseline Characteristics
Hypothesis: Low levels of LDL-C can be beneficial in terms of plaque reduction. The magnitude of the benefit may be greater in patients with acute MI, suggesting that an early approach may reduce the adverse outcomes. A pragmatic effectiveness trial can efficiently evaluate this hypothesis.
Aims: To evaluate the effectiveness of early administration of evolocumab, a PCSK9 inhibitor, combined with usual care in reducing recurrent MI.
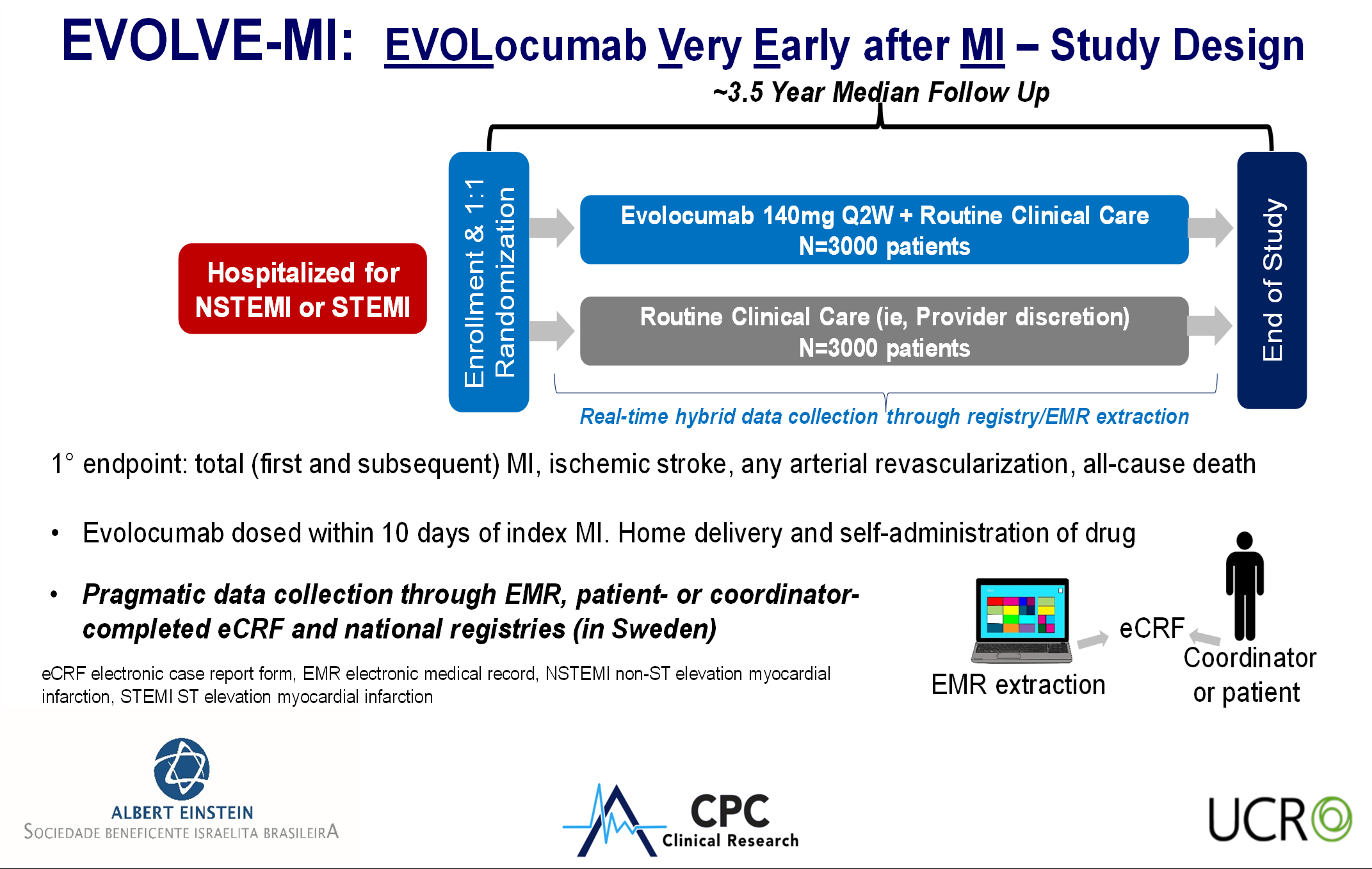
Methods: EVOLVE-MI is an international, pragmatic, randomized trial (Panel A) with streamlined activities including remote study visits, data collected directly from registries and electronic medical records, de-centralized adjudication, patient self-reporting, and drug distribution through pharmacy cards. A total of 114 sites across United States, Brazil, and Sweden enrolled patients within 10 days of MI. Patients were randomized 1:1 to evolocumab with usual care or usual care alone (high-intensity statins or moderate-intensity statins with ezetimibe). No qualifying LDL-C or background lipid modifying therapy was required. The primary efficacy outcome is the composite of total (first and recurrent) MI, ischemic stroke, arterial revascularization, or death with a planned number of total events of 1891. Enrollment was completed in May 2024.
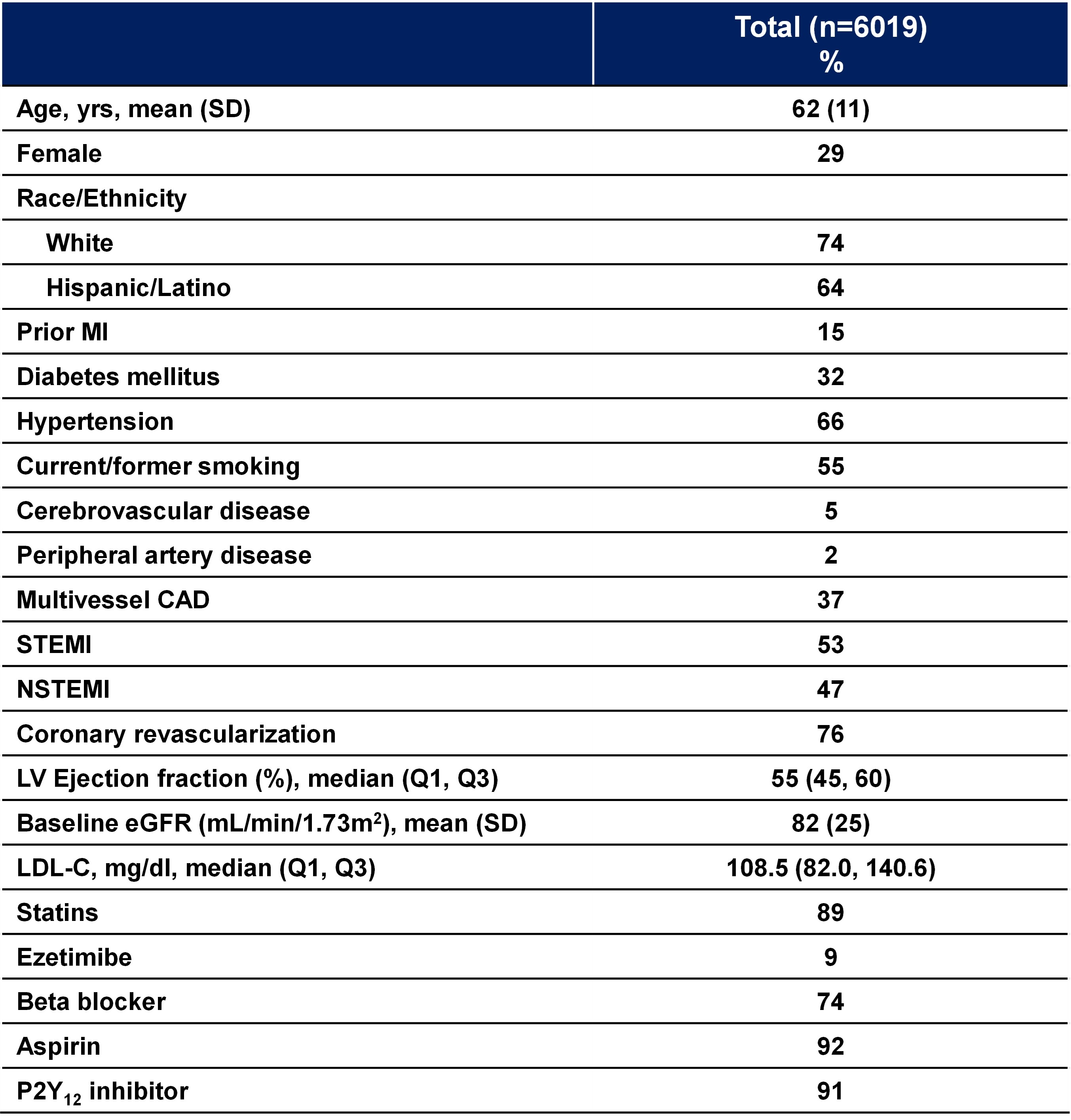
Results: The study randomized 6019 patients (Panel B). Patients have a mean age of 62 years, with 32% having diabetes mellitus, 66% having hypertension, and 55% being current or former smokers. In addition, 15% have prior MI, 37% have multivessel CAD, and 5% and 2% having known cerebrovascular disease or peripheral artery disease, respectively. The presenting syndrome was STEMI in 53% and NSTEMI in 47% of patients. Baseline ejection fraction was 55% and baseline LDL-C was 108 mg/dL. For the index event, 92% and 91% received aspirin and P2Y12 inhibition, respectively. Statins were used 89% and ezetimibe in 9% of the enrolled patients.
Conclusion: This pragmatic randomized trial is evaluating the hypothesis that early administration of potent LDL-C lowering with evolocumab in patients with MI improves outcomes. The trial is ongoing to obtain the planned number of events to determine if this strategy reduces cardiovascular events.
- Bonaca, Marc ( CPC Clinical Research , Aurora , Colorado , United States )
- Wang, Huei ( AMGEN INC , Thousand Oaks , California , United States )
- Wu, You ( AMGEN INC , Thousand Oaks , California , United States )
- Cyrille, Marcoli ( AMGEN INC , Thousand Oaks , California , United States )
- Cannon, Christopher ( Brigham and Womens Hospital , Boston , Massachusetts , United States )
- Hess, Connie ( CPC Clinical Research , Aurora , Colorado , United States )
- Tavares, Caio ( Albert Einstein , São Paulo , Brazil )
- James, Stefan ( Uppsala Clinical Research Center , Uppsala , Sweden )
- Oldgren, Jonas ( Uppsala Clinical Research Center , Uppsala , Sweden )
- Bhatt, Deepak ( Mount Sinai , New York , New York , United States )
- Abbott, Jinnette ( Brown University Health Cardiovascular Institute and Alpert Medical School of Brown University , Providence , Rhode Island , United States )
- Mehran, Roxana ( Icahn School of Med. at Mount Sinai , New York , New York , United States )
- Berwanger, Otavio ( The George Institute for Global Health and Imperial College , London , United Kingdom )
Meeting Info:
Session Info:
Saturday, 11/08/2025 , 01:45PM - 02:55PM
Moderated Digital Poster Session
More abstracts on this topic:
Manalo Kathryn, Tieliwaerdi Xiarepati, Jackson Megan, Arrigo Alexis, Mascara Mariah, Maharjan Srijana, Gadani Mrudula
A Perfect Storm: Simultaneous Pulmonary Embolism, STEMI, and Stroke via Paradoxical Embolism in a Hospitalized Patient on DVT ProphylaxisKhan Abdul Allam, Thukral Jatin, Elgabry Ibrahim, Lamp Garron
More abstracts from these authors:
Monguillon Victorien, O'donoghue Michelle, Lopez J. Antonio, Rosenson Robert, Ran Xinhui, Wang Jingying, Wang Huei, Wu You, Kassahun Helina, Sabatine Marc
Discussant: CLEAR SYNERGYBonaca Marc