Final ID: MP31
Hyperglycemic adipocyte-derived exosomes aggravate oxidative stress, lipid peroxidation and mitochondrial dysfunction of brain microvascular endothelial cells in ischemic stroke
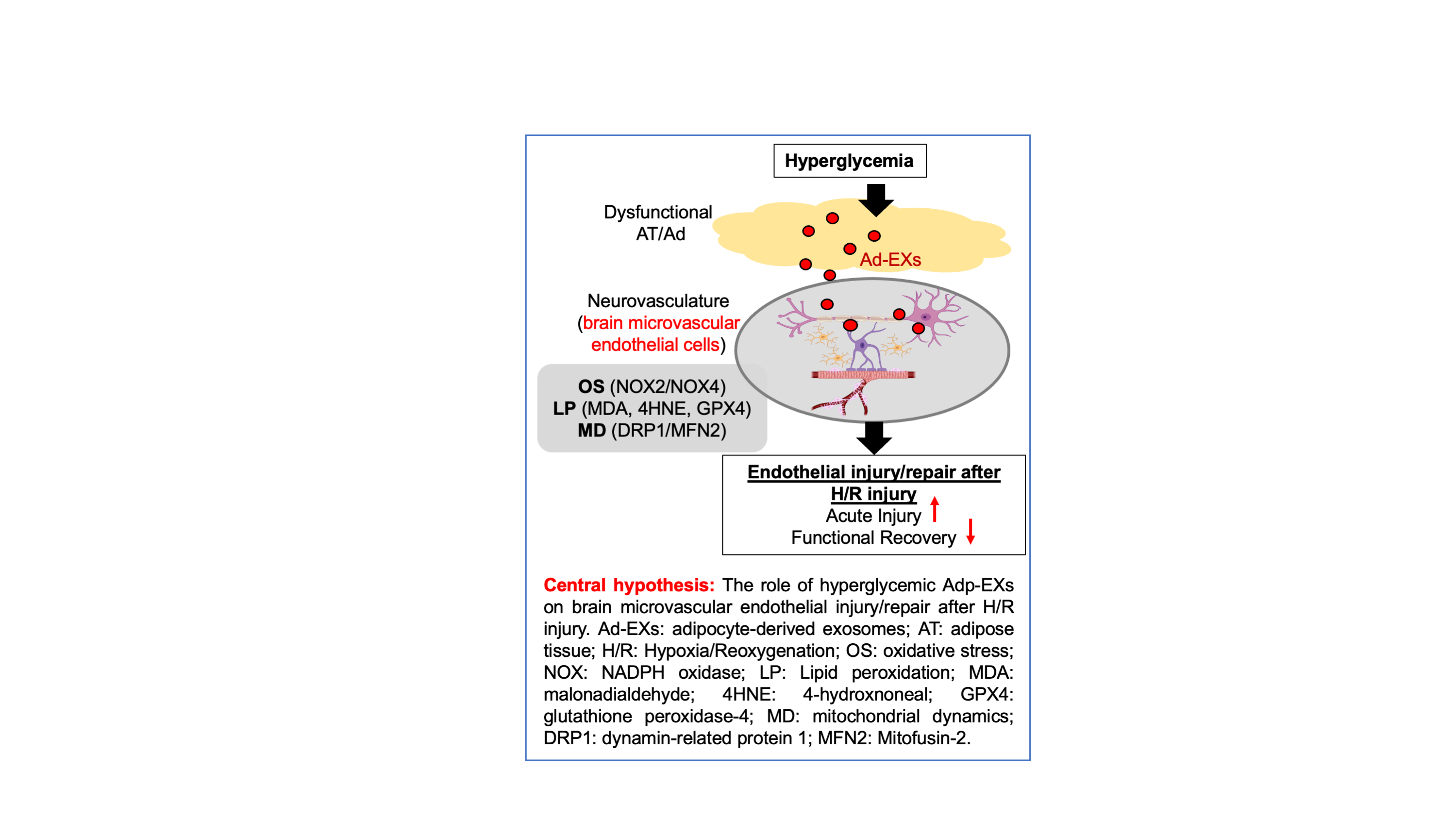
Abstract Body (Do not enter title and authors here): Background: Type 2 Diabetes Mellitus, a risk factor for ischemic stroke (IS), is characterized by hyperglycemia and adipose tissue (AT) dysfunction. AT excert endocrine and paracrine functions by its secretome, including exosomes. Our previous studies showed that high glucose (HG) promotes release of exosomes from adipocytes (Ad-EXs) and their altered cargo. Here, we examined the effects of hyperglycemic Ad-EXs on brain microvascular endothelial cells (BMECs) in IS. Hypothesis: Hyperglycemic Ad-EXs aggravate BMECs function via inducing oxidative stress, lipid peroxidation and mitochondrial dysfunction during IS. Methods: Primary human adipocytes were cultured in normal-glucose(NG) or HG(25mM) media to isolate NG-Ad-EXs or HG-Ad-EXs, followed by their co-incubation with primary human BMECs (HBMECs) for 48 hrs post Hypoxia/Reoxygenation(H/R) injury stimulating IS in-vitro. The HBMEC viability, migration, tube formation, membrane protentional (MMP) and ATP production assay were performed. The reactive oxygen species (ROS) levels were measured by dihydroethidium and malondialdehyde assays. Western blot analyzed expression of OS (NOX2/4), lipid peroxidation (MDA, 4HNE, GPX4) and mitochondrial dysfunction (DRP-1, Mfn2). For the In-vivo study, middle cerebral occlusion surgery was performed to induce IS in control mice, followed by injection of Ad-EXs (1x1011EX/mL) isolated from control or diabetic (db/db) mice adipocytes (cAd-EXs or dAd-EXs) via tail vein. The infraction size was measured by Cresyl Violet staining and neurological function was assessed by corner and adhesive tests. Results: In-vitro, HG-Ad-EXs decreased HBMEC viability, migration (~2x), and tube formation (~2x) abilities vs NG-Ad-EXs (p<0.05). HG-Ad-EXs impaired mitochondrial function by increasing ROS production (~1.5x) but decreasing MMP (~1.5x) and ATP production (~0.5x). Mechanistically, HG-Ad-EXs significantly upregulated NOX2/4, MDA, 4HNE and downregulated GPX4, increased DRP-1 and decreased Mfn2. In-vivo, injected Ad-EXs crossed the blood-brain barrier and incorporated into brain ECs in peri-infarct area on day 2. The infarct size was enlarged, and both contact/removal times were extended in dAd-EXs group (p<0.05, vs. vehicle and cAd-EXs). Conclusion: Our data suggest that diabetic Ad-EXs exacerbate endothelial dysfunction under H/R by triggering oxidative stress, lipid peroxidation and mitochondrial dysfunction, which potentially contributes to exacerbated neurological function after IS.
More abstracts on this topic:
Analysis of Polarization Changes in Ascending Aortic Endothelial Cells Induced by Aortic Valve Regurgitation
Tamagawa Yuki, Miyagawa Shigeru, Kawamura Takuji, Harada Akima, Miyake Keisuke, Kawamura Ai, Kido Takashi, Yamashita Kizuku, Shimamura Kazuo, Saito Shunsuke
An improved design and matching algorithm for dementia risk assessment following first-ever stroke in population-based cohorts in Europe and the U.S.AWaziry Reem