Final ID: MP423
The metabolome is preserved in failing mouse hearts but shifts under additional stress
Abstract Body (Do not enter title and authors here): The healthy heart is omnivorous as it readily utilizes fatty acids, glucose, lactate, pyruvate, ketone bodies, and amino acids. This adaptation enables the heart to metabolize alternative fuels when the preferred fatty acids for maximal ATP production cannot be utilized due to decreased cardiac efficiency. It is well established that the failing human heart ultimately becomes metabolically insufficient as it gradually shifts to fuels such as ketone bodies and fails to generate enough ATP to compensate for the energy deficit. While mouse HF models are being utilized to elucidate underlying pathomechanisms and explore metabolic therapy targets, the metabolome of the failing mouse heart is not well characterized. Thus, using stable isotope-labeled metabolites, we sought to characterize the global metabolome and fuel utilization in failing mice myocardia.
We hypothesize the metabolome and fuel utilization of the failing mice heart will change substantially like failing human hearts.
To assess the metabolic phenotype of the failing mice heart, we induced HFrEF in mice with TAC/MI surgeries, followed by echocardiography after 4 weeks to assess systolic functions and morphometrics. Next, cocktails of isotope-labeled metabolites (glucose, lactate, β-OHB, glutamine, and valine) were infused intravenously for 2 hr while arterial blood was collected at different time points. Alternatively, isoproterenol (90 ng/kg/min) was added to the cocktail to mimic ambulatory heart rates while metabolites were being infused.
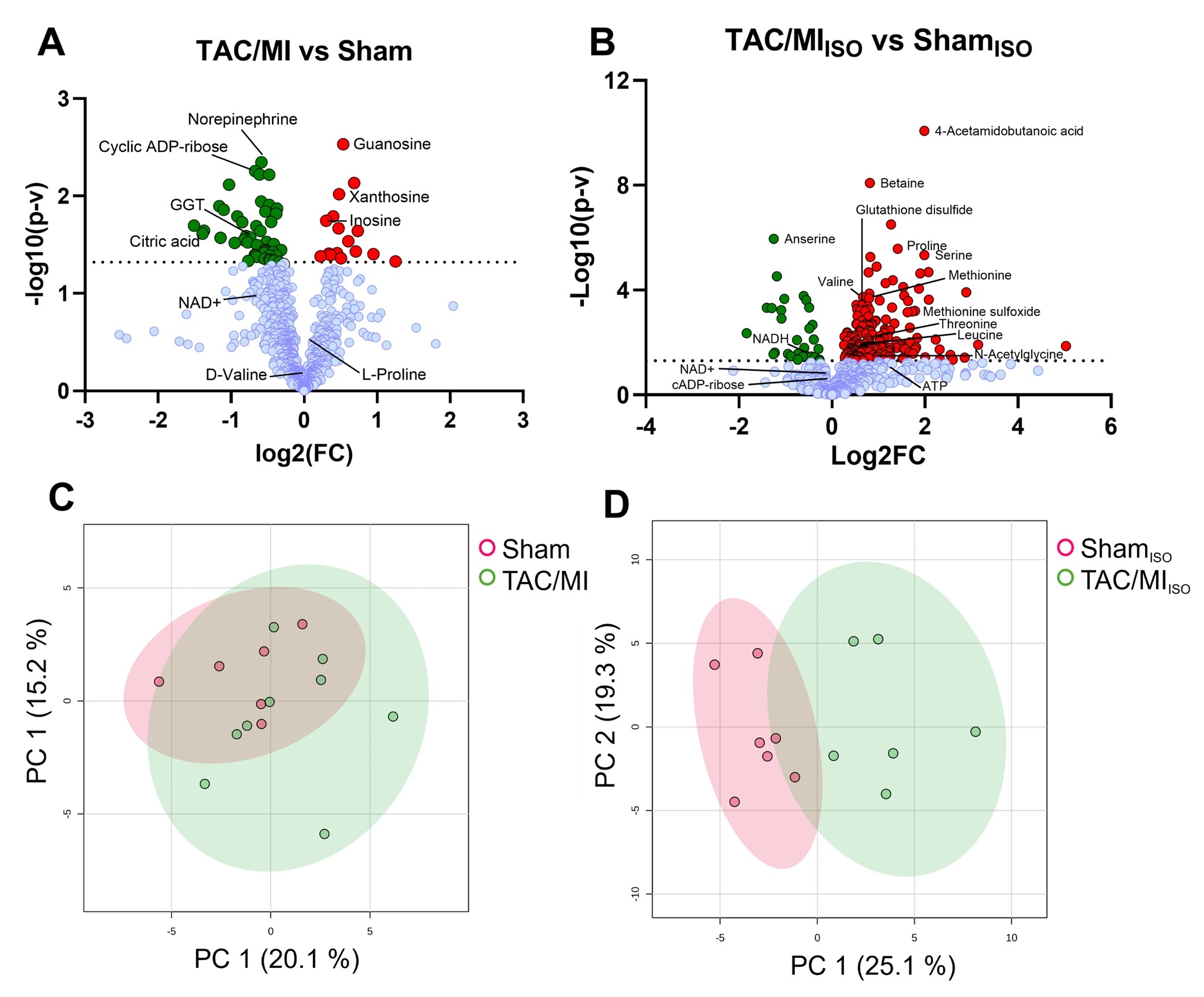
Intriguingly, the metabolome of the TAC/MI with reduced LVEF compared to the Sham showed only 7.2% of myocardial metabolite levels altered; principal component analysis (PCA) showed no overt change in the metabolome. Similarly, isotope tracing data showed no differences in metabolite enrichment and fuel utilization in the myocardia. However, TAC/MI drastically altered the response to additional stress with isoproterenol, with 34.9% of metabolites changed in myocardial metabolomics between TAC/MI and Sham. Also, PCA showed a significantly diverging metabolic profiling between the failing and normal hearts from TAC/MI and Sham mice, respectively – representing similar observations in failing human hearts.
We show for the first time that metabolome is preserved in failing mouse hearts but shifts under stress, thus warranting further investigation into the dynamics of the metabolic profile of the failing heart for insights into developing metabolic therapies for HF.
We hypothesize the metabolome and fuel utilization of the failing mice heart will change substantially like failing human hearts.
To assess the metabolic phenotype of the failing mice heart, we induced HFrEF in mice with TAC/MI surgeries, followed by echocardiography after 4 weeks to assess systolic functions and morphometrics. Next, cocktails of isotope-labeled metabolites (glucose, lactate, β-OHB, glutamine, and valine) were infused intravenously for 2 hr while arterial blood was collected at different time points. Alternatively, isoproterenol (90 ng/kg/min) was added to the cocktail to mimic ambulatory heart rates while metabolites were being infused.
Intriguingly, the metabolome of the TAC/MI with reduced LVEF compared to the Sham showed only 7.2% of myocardial metabolite levels altered; principal component analysis (PCA) showed no overt change in the metabolome. Similarly, isotope tracing data showed no differences in metabolite enrichment and fuel utilization in the myocardia. However, TAC/MI drastically altered the response to additional stress with isoproterenol, with 34.9% of metabolites changed in myocardial metabolomics between TAC/MI and Sham. Also, PCA showed a significantly diverging metabolic profiling between the failing and normal hearts from TAC/MI and Sham mice, respectively – representing similar observations in failing human hearts.
We show for the first time that metabolome is preserved in failing mouse hearts but shifts under stress, thus warranting further investigation into the dynamics of the metabolic profile of the failing heart for insights into developing metabolic therapies for HF.
More abstracts on this topic:
A Novel Machine Learning Strategy to Integrate Multi-Omics Data and Detect Genomic Loci and Gene-Environment Interactions for LDL Cholesterol
Li Changwei, Zhang Ruiyuan, Sun Yixi, Chen Jing, Wang Tao, Bazzano Lydia, Kelly Tanika, He Jiang
A Metabolomic Study of Cardiac Dysfunction in HyperglycemiaYoshida Yilin, Qi Qibin, Cheng Susan, Kaplan Robert, Rodriguez Carlos, Shah Amil, Yu Bing, Nguyen Ngoc Quynh, Moon Eun Hye, Casey Rebholz, Skali Hicham, Arthur Victoria, Echouffo Justin, Ballantyne Christie, Selvin Elizabeth