Final ID: BCVS5
Macrophage-Mediated Interleukin-6 Signaling Drives Ryanodine Receptor-2 Calcium Leak in Postoperative Atrial Fibrillation
Abstract Body (Do not enter title and authors here): Postoperative atrial fibrillation (poAF) is self-limited AF that occurs days after cardiac surgery in one-third of patients. The degree of perioperative increase in systemic inflammation, particularly involving interleukin (IL)-6, correlates with poAF risk. However, no studies to-date have identified the cell types involved nor mechanistically linked IL-6 signaling to fundamental arrhythmia mechanisms.
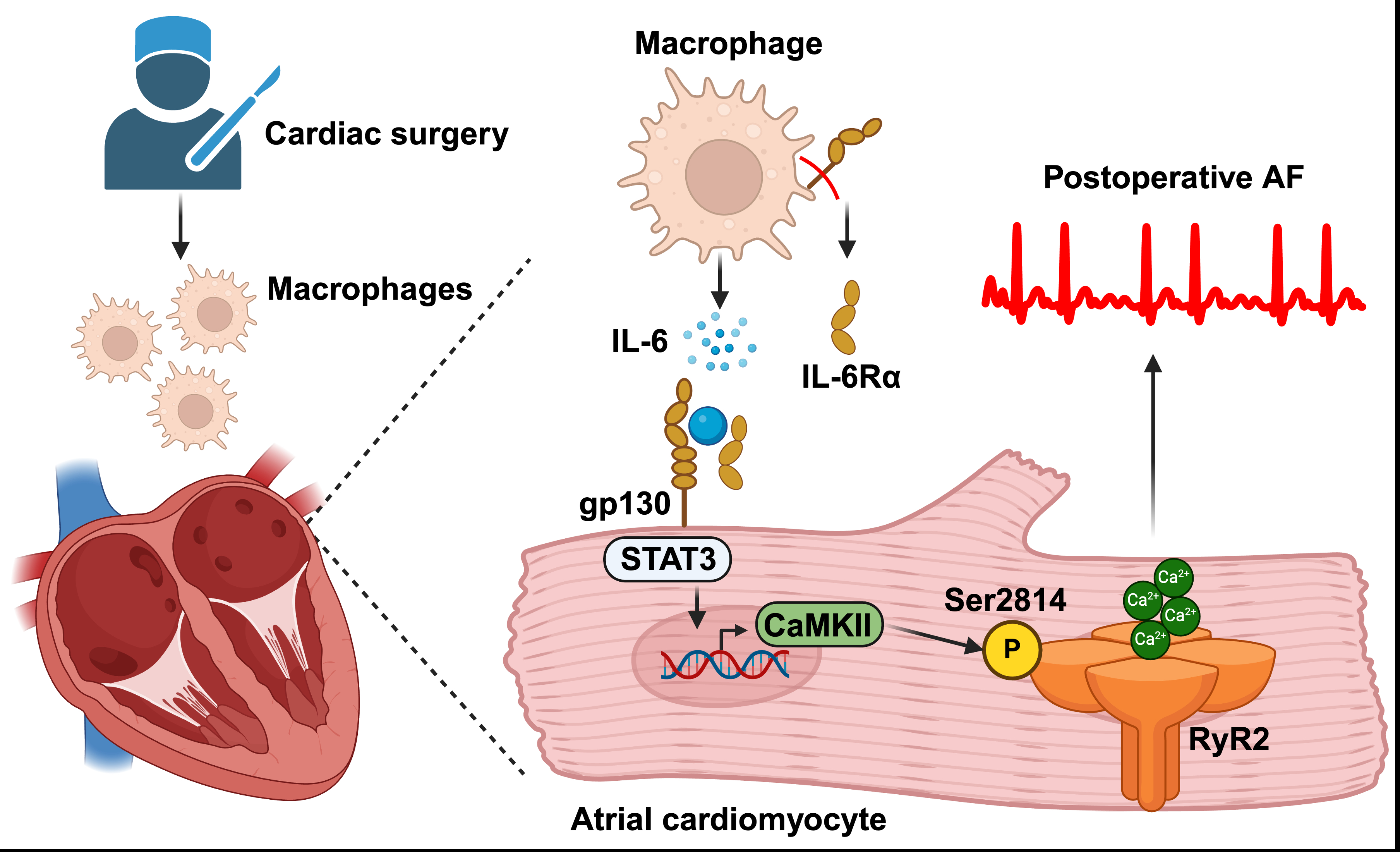
To study poAF, we developed a mouse model consisting of atrial pericardiectomy and transient aortic cross-clamping followed by intracardiac burst pacing on postoperative day three to assess poAF inducibility. Single-cell RNA sequencing of atrial non-myocytes revealed macrophages to be the most prominently altered atrial cell type – increased 2.4-fold (P<0.001) in mice with versus without poAF. Pseudotime trajectory analyses revealed IL-6 to be the top cytokine altered in macrophages, which we confirmed in the atria of mice with poAF and pericardial fluid of human patients 72-h after open-heart surgery. Indeed, both macrophage depletion and conditional knockout of IL-6Ra in macrophages decreased poAF inducibility by 5.2-fold (P=0.048) and 5.5-fold (P=0.027), respectively. Downstream inhibition using an FDA-approved STAT3 inhibitor also rescued poAF (6.0-fold decrease, P=0.022). In isolated atrial cardiomyocytes (ACMs), IL-6 challenge led to a 6.3-fold (P<0.001) increase in ryanodine receptor-2 (RyR2)-dependent calcium (Ca2+) sparks. Indeed, RyR2 phosphorylation at Ser2814, the target of Ca2+/calmodulin-dependent protein kinase II (CaMKII), was increased in the atria of mice with poAF, and CaMKII inhibition was sufficient to rescue arrhythmogenic Ca2+sparks in ACMs from mice with poAF. We corroborated our findings in human ACMs and atrial tissue to demonstrate translational relevance.
Taken together, we use an integrated approach incorporating mouse and human biochemical, functional, and single-cell sequencing data to demonstrate that infiltrating atrial macrophages, specifically IL-6Ra from macrophages, are fundamentally required for poAF development (Fig. 1). Molecularly, enhanced atrial IL-6 signaling led to downstream arrhythmogenic RyR2 dysfunction through CaMKII. Our findings portend therapeutic utility by providing mechanistic evidence that targeting the IL-6 axis may be used for poAF prevention and treatment in a clinically amenable timeframe.
To study poAF, we developed a mouse model consisting of atrial pericardiectomy and transient aortic cross-clamping followed by intracardiac burst pacing on postoperative day three to assess poAF inducibility. Single-cell RNA sequencing of atrial non-myocytes revealed macrophages to be the most prominently altered atrial cell type – increased 2.4-fold (P<0.001) in mice with versus without poAF. Pseudotime trajectory analyses revealed IL-6 to be the top cytokine altered in macrophages, which we confirmed in the atria of mice with poAF and pericardial fluid of human patients 72-h after open-heart surgery. Indeed, both macrophage depletion and conditional knockout of IL-6Ra in macrophages decreased poAF inducibility by 5.2-fold (P=0.048) and 5.5-fold (P=0.027), respectively. Downstream inhibition using an FDA-approved STAT3 inhibitor also rescued poAF (6.0-fold decrease, P=0.022). In isolated atrial cardiomyocytes (ACMs), IL-6 challenge led to a 6.3-fold (P<0.001) increase in ryanodine receptor-2 (RyR2)-dependent calcium (Ca2+) sparks. Indeed, RyR2 phosphorylation at Ser2814, the target of Ca2+/calmodulin-dependent protein kinase II (CaMKII), was increased in the atria of mice with poAF, and CaMKII inhibition was sufficient to rescue arrhythmogenic Ca2+sparks in ACMs from mice with poAF. We corroborated our findings in human ACMs and atrial tissue to demonstrate translational relevance.
Taken together, we use an integrated approach incorporating mouse and human biochemical, functional, and single-cell sequencing data to demonstrate that infiltrating atrial macrophages, specifically IL-6Ra from macrophages, are fundamentally required for poAF development (Fig. 1). Molecularly, enhanced atrial IL-6 signaling led to downstream arrhythmogenic RyR2 dysfunction through CaMKII. Our findings portend therapeutic utility by providing mechanistic evidence that targeting the IL-6 axis may be used for poAF prevention and treatment in a clinically amenable timeframe.
More abstracts on this topic:
A comparison of the efficacy of initial high energy versus initial low energy biphasic shocks for cardioversion of atrial fibrillation and atrial flutter – a real-life experience
Alampoondi Venkataramanan Sai Vikram, Vunnam Ramarao, Voruganti Dinesh, Tsai Shane
A mechanism whereby SGLT2 inhibitor dapagliflozin reverses cardiac diastolic dysfunction in a model of HFpEFLiu Man, Liu Hong, Kang Gyeoung-jin, Kim Eunji, Neumann Mitchell, Johnson Madeline, Murikinati Ruthvika, Dudley Samuel