Final ID: 4171296
Effects of Intensive Blood Pressure Control in Patients with Type 2 Diabetes
Abstract Body (Do not enter title and authors here): Hypothesis and Purpose: The optimal control target for systolic blood pressure (BP) in type 2 diabetes is unknown due to limited and inconsistent findings from clinical trials. We aimed to test whether an intensive treatment strategy to target systolic BP of <120 mmHg is more effective than a standard treatment strategy to target systolic BP of <140 mmHg in reducing the risk of major cardiovascular disease (CVD) over a follow-up period of up to 5 years among patients with type 2 diabetes.
Study Design and Methods: The Blood Pressure Control Target in Diabetes (BPROAD) Study is a multi-center, open-label, parallel-group, randomized controlled clinical trial (ClinicalTrials.gov Identifier: NCT03808311).
Sample Size: 12,821 participants from 145 study sites located in 25 provinces across mainland China.
Population Studied: Type 2 diabetes patients aged ≥50 years with elevated systolic BP and increased risk of CVD.
Intervention(s): Systolic BP <120 mmHg for the intensive treatment group and systolic BP <140 mmHg for the standard treatment group.
Power Calculations: We had 90% statistical power to detect a 20% risk reduction for CVD events associated with the intervention at a two-sided significance level of 0.05.
Primary End Point: Major cardiovascular events defined as the composite endpoint of the first occurrence of non-fatal stroke, non-fatal myocardial infarction, hospitalized or treated heart failure, and cardiovascular deaths.
Key Secondary End Points: Individual components of the composite primary end point, all-cause mortality, and an expanded end point including the composite primary outcome and all-cause mortality.
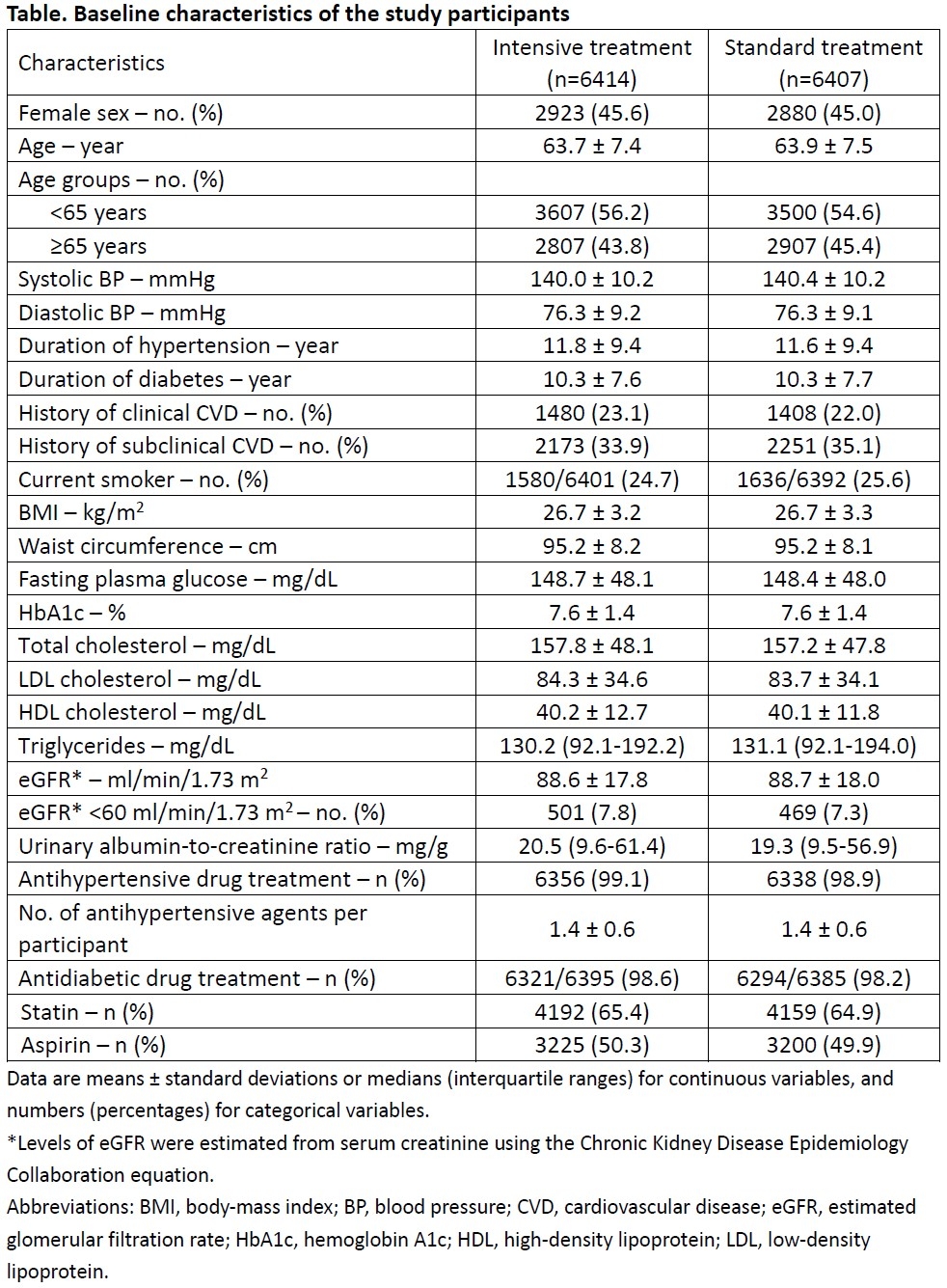
Main Results: 6,414 participants were enrolled in the intensive treatment group and 6,407 participants in the standard treatment group between February 2019 and December 2021. The mean age of BPROAD participants was 63.8 (standard deviation, 7.5) years. 45.3% were women. 22.5% had a self-reported history of CVD. The median follow-up duration was 4.2 years. The mean systolic BP at the 48-month visit was 120.6 mmHg in the intensive treatment group and 132.1 mmHg in the standard treatment group. The primary outcome events occurred in 393 participants (1.65% per year) in the intensive treatment group and 492 participants (2.09% per year) in the standard treatment group (hazard ratio: 0.79; 95% confidence interval: 0.69 to 0.90; p=0.0005). Details of cardiovascular outcomes and safety findings will be presented.
Study Design and Methods: The Blood Pressure Control Target in Diabetes (BPROAD) Study is a multi-center, open-label, parallel-group, randomized controlled clinical trial (ClinicalTrials.gov Identifier: NCT03808311).
Sample Size: 12,821 participants from 145 study sites located in 25 provinces across mainland China.
Population Studied: Type 2 diabetes patients aged ≥50 years with elevated systolic BP and increased risk of CVD.
Intervention(s): Systolic BP <120 mmHg for the intensive treatment group and systolic BP <140 mmHg for the standard treatment group.
Power Calculations: We had 90% statistical power to detect a 20% risk reduction for CVD events associated with the intervention at a two-sided significance level of 0.05.
Primary End Point: Major cardiovascular events defined as the composite endpoint of the first occurrence of non-fatal stroke, non-fatal myocardial infarction, hospitalized or treated heart failure, and cardiovascular deaths.
Key Secondary End Points: Individual components of the composite primary end point, all-cause mortality, and an expanded end point including the composite primary outcome and all-cause mortality.
Main Results: 6,414 participants were enrolled in the intensive treatment group and 6,407 participants in the standard treatment group between February 2019 and December 2021. The mean age of BPROAD participants was 63.8 (standard deviation, 7.5) years. 45.3% were women. 22.5% had a self-reported history of CVD. The median follow-up duration was 4.2 years. The mean systolic BP at the 48-month visit was 120.6 mmHg in the intensive treatment group and 132.1 mmHg in the standard treatment group. The primary outcome events occurred in 393 participants (1.65% per year) in the intensive treatment group and 492 participants (2.09% per year) in the standard treatment group (hazard ratio: 0.79; 95% confidence interval: 0.69 to 0.90; p=0.0005). Details of cardiovascular outcomes and safety findings will be presented.
More abstracts on this topic:
A Daily Diary Examination of the Associations of Adverse Childhood Experiences, Anticipated Discrimination, and Blood Pressure among Sexual and Gender Minority Adults
A Longitudinal 20-year Analysis Indicates Acceleration of Cardiometabolic Comorbidities on Dementia Risk
Pardee Lisa, Bochicchio Lauren, Caceres Billy
A Longitudinal 20-year Analysis Indicates Acceleration of Cardiometabolic Comorbidities on Dementia Risk
Lihua Huang, Danish Muhammad, Auyeung Tw, Jenny Lee, Kwok Timothy, Abrigo Jill, Wei Yingying, Lo Cecilia, Fung Erik