Final ID: 4170996
Natural Language Processing to Adjudicate Heart Failure Hospitalizations in Global Clinical Trials
Background: Medical record review by a physician clinical events committee (CEC) is the gold standard for adjudicating heart failure hospitalizations (HFH) in clinical trials but is labor-intensive. Automated adjudication by natural language processing (NLP) could improve efficiency, but has not been validated in global trials.
Methods: We developed a model for automated NLP-based HFH adjudication (“HF-NLP”) that integrates two complementary approaches: a Clinical Longformer model trained in the INVESTED, PARADISE-MI, and PARAGON-HF trials, and a GPT4o prompt that identifies HF criteria (signs, symptoms, and treatment) and quotes supporting sentences. The HF-NLP model generates a binary HFH adjudication and an ordinal five-category score indicating increasing probability of HFH. In the DELIVER randomized trial, which compared dapagliflozin to placebo in 6063 patients with heart failure with mildly reduced or preserved ejection fraction, we evaluated agreement between HF-NLP and human CEC adjudications and compared the estimated effect of dapagliflozin on HFH using HF-NLP versus CEC adjudication.
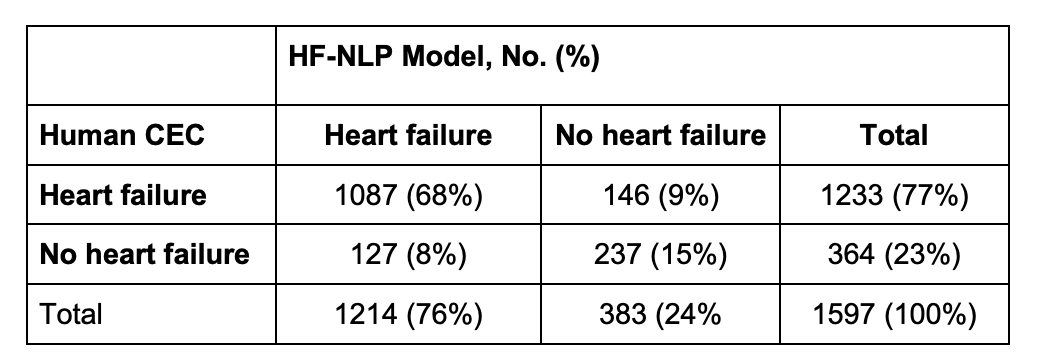
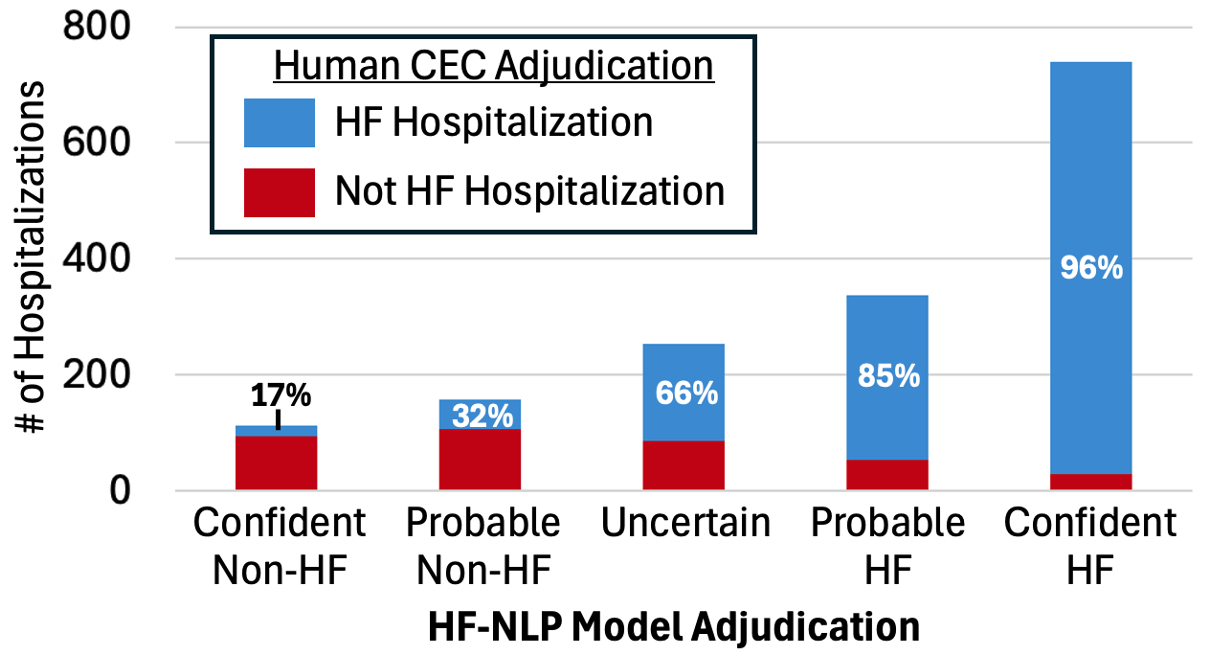
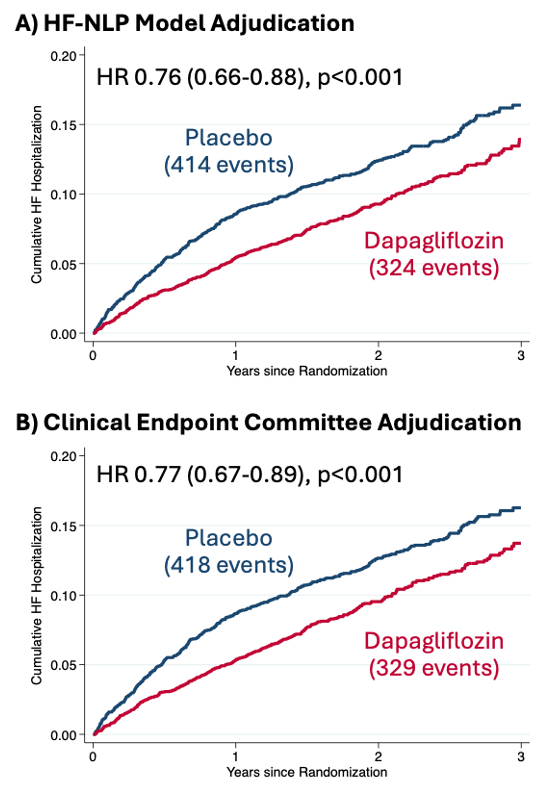
Results: The DELIVER CEC adjudicated HFH in 1233/1597 (77%) of investigator-reported events. The HF-NLP adjudication agreed with the CEC in 83% of events (Figure 1). The proportion of events adjudicated as HFH by the CEC increased across the HF-NLP ordinal categories: 17% in confident non-HFH, 32% in probable non-HFH, 66% in uncertain, 85% in probable HFH, and 96% in confident HFH (Figure 2). A strategy of human review for events that HF-NLP deemed uncertain would have achieved 91% agreement with the CEC while reducing adjudication workload by 84%. The effect of dapagliflozin on HFH was similar with HF-NLP adjudication (hazard ratio [HR] 0.76 [95% CI 0.66-0.88]) compared to CEC adjudication (HR 0.77 [95% CI 0.67-0.89]) (Figure 3).
Conclusion: Automated adjudication of HFH by the HF-NLP model demonstrated substantial agreement with the physician CEC in the DELIVER trial and yielded a similar estimate of dapagliflozin efficacy. NLP adjudication could improve the efficiency of global clinical trials while preserving accuracy and interpretability.
- Cunningham, Jonathan ( Brigham and Womens Hospital , Chestnut Hill , Massachusetts , United States )
- Vardeny, Orly ( Minneapolis VA Health Care System , Minneapolis , Minnesota , United States )
- Lewis, Eldrin ( STANFORD UNIVERSITY , Palo Alto , California , United States )
- Pfeffer, Marc ( BRIGHAM and WOMENS HOSPITAL , Boston , Massachusetts , United States )
- Jhund, Pardeep ( UNIVERSITY OF GLASGOW , Glasgow , United Kingdom )
- Desai, Akshay ( BRIGHAM WOMENS HOSPITAL , Boston , Massachusetts , United States )
- Mcmurray, John ( BHF CARDIOVASCULAR RESEARCH CENTRE , Glasgow , United Kingdom )
- Ellinor, Patrick ( Massachusetts General Hospital , Boston , Massachusetts , United States )
- Ho, Jennifer ( Harvard Medical School , Newton , Massachusetts , United States )
- Solomon, Scott ( , Boston , Massachusetts , United States )
- Marti Castellote, Pablo-miki ( BWH, Harvard Medical School , Boston , Massachusetts , United States )
- Reeder, Christopher ( Broad Institute , Cambridge , Massachusetts , United States )
- Singh, Pulkit ( Broad Institute , Cambridge , Massachusetts , United States )
- Lau, Emily ( Massachusetts General Hospital , Jamaica Plain , Massachusetts , United States )
- Khurshid, Shaan ( Massachusetts General Hospital , Boston , Massachusetts , United States )
- Batra, Puneet ( Broad Institute , Cambridge , Massachusetts , United States )
- Lubitz, Steven ( Massachusetts General Hospital , Boston , Massachusetts , United States )
- Maddah, Mahnaz ( Broad Institute , Cambridge , Massachusetts , United States )
Meeting Info:
Session Info:
Featured Science: Health Technology and the Future of Clinical Trials
Saturday, 11/16/2024 , 01:30PM - 02:45PM
Featured Science
More abstracts on this topic:
Jiang Meng, Guo Xinning
A National Analysis Of Heart Donation After Circulatory Death In The United StatesFiroz Ahad, Ebong Imo, Cadeiras Martin, Jimenez Shirin
More abstracts from these authors:
Marti Castellote Pablo-miki, Desai Akshay, Ellinor Patrick, Ho Jennifer, Mcmurray John, Pfeffer Marc, Solomon Scott, Cunningham Jonathan, Badrouchi Samarra, Claggett Brian, Xu Dongchu, Maddah Mahnaz, Khurshid Shaan, Vardeny Orly, Lewis Eldrin, Jhund Pardeep
Artificial Intelligence to Extract Structured Details from Unstructured Medical Records in a Global Heart Failure TrialBadrouchi Samarra, Mcgrath Martina, Mc Causland Finnian, Mcmurray John, Solomon Scott, Cunningham Jonathan, Marti Castellote Pablo-miki, Foa' Alberto, Claggett Brian, Xu Dongchu, Desai Akshay, Ho Jennifer, Ellinor Patrick, Jhund Pardeep