Final ID: 4170664
Efficacy of a Glucagon-Like Peptide-1 Agonist in Patients undergoing Coronary Artery Bypass Grafting or Aortic Valve Replacement – a randomized clinical trial
Abstract Body (Do not enter title and authors here): Hypothesis and Purpose:
Administering the GLP-1 analog exenatide prior to cardio-pulmonary bypass-assisted (CPB) coronary artery bypass grafting (CABG) and/or aortic valve replacement (AVR) is hypothesized to reduce mortality and morbidity related to heart, brain, and kidney injury.
Study Design and Methods:
The GLORIOUS trial (NCT02673931) was an investigator-initiated, single-center, 2-by-2, randomized clinical trial. The two interventions were considered independent, and this abstract reports on one of those interventions. The other intervention (restrictive vs. liberal oxygenation during cardio-pulmonary bypass) will be reported separately.
Sample Size:
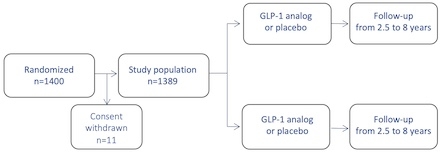
1400 patients.
Population Studied:
Adult patients scheduled for elective or subacute CPB-assisted CABG and/or surgical AVR.
Intervention:
A 6 hour and 15 minutes infusion of either 17.4 μg of exenatide (Byetta®, Lilly) or placebo initiated prior to the induction of anesthesia in a double-blind fashion.
Power Calculations:
No interaction between the two interventions was expected, and the sample size estimation did not account for effect modification. With an alpha-level of 0.05 and a power of 0.8, the trial aimed to demonstrate a 25% reduction in the primary endpoint, assuming an event rate of 23% (i.e., 323 events) in 1400 patients. The trial was event-driven and follow-up has been concluded after 323 total events were reached.
Primary End Points:
Time to the first occurrence of any of the co-primary endpoints during follow-up, including death, renal failure requiring renal replacement therapy, stroke, new-onset heart failure, or any re-admission for heart failure.
Secondary End Points:
Incidence of predefined safety endpoints during the index admission, such as surgical site infection, hypoglycemia, pancreatitis, a relative reduction of ejection fraction by 50% compared to baseline, reoperation for bleeding and any cause, and post-surgery myocardial infarction. Additionally, re-admission for cardiovascular causes within 12 months was monitored.
Outcome:
From February 2016 to December 2021, 1400 patients were enrolled in the study, with follow-up concluding in June 2024. The study will be un-blinded on August 27, 2024. The main results for the two interventions of the GLORIOUS trial have been submitted as separate abstracts for AHA’s late-breaking session. If accepted, the two primary articles will be submitted for simultaneous publication.
Administering the GLP-1 analog exenatide prior to cardio-pulmonary bypass-assisted (CPB) coronary artery bypass grafting (CABG) and/or aortic valve replacement (AVR) is hypothesized to reduce mortality and morbidity related to heart, brain, and kidney injury.
Study Design and Methods:
The GLORIOUS trial (NCT02673931) was an investigator-initiated, single-center, 2-by-2, randomized clinical trial. The two interventions were considered independent, and this abstract reports on one of those interventions. The other intervention (restrictive vs. liberal oxygenation during cardio-pulmonary bypass) will be reported separately.
Sample Size:
1400 patients.
Population Studied:
Adult patients scheduled for elective or subacute CPB-assisted CABG and/or surgical AVR.
Intervention:
A 6 hour and 15 minutes infusion of either 17.4 μg of exenatide (Byetta®, Lilly) or placebo initiated prior to the induction of anesthesia in a double-blind fashion.
Power Calculations:
No interaction between the two interventions was expected, and the sample size estimation did not account for effect modification. With an alpha-level of 0.05 and a power of 0.8, the trial aimed to demonstrate a 25% reduction in the primary endpoint, assuming an event rate of 23% (i.e., 323 events) in 1400 patients. The trial was event-driven and follow-up has been concluded after 323 total events were reached.
Primary End Points:
Time to the first occurrence of any of the co-primary endpoints during follow-up, including death, renal failure requiring renal replacement therapy, stroke, new-onset heart failure, or any re-admission for heart failure.
Secondary End Points:
Incidence of predefined safety endpoints during the index admission, such as surgical site infection, hypoglycemia, pancreatitis, a relative reduction of ejection fraction by 50% compared to baseline, reoperation for bleeding and any cause, and post-surgery myocardial infarction. Additionally, re-admission for cardiovascular causes within 12 months was monitored.
Outcome:
From February 2016 to December 2021, 1400 patients were enrolled in the study, with follow-up concluding in June 2024. The study will be un-blinded on August 27, 2024. The main results for the two interventions of the GLORIOUS trial have been submitted as separate abstracts for AHA’s late-breaking session. If accepted, the two primary articles will be submitted for simultaneous publication.
More abstracts on this topic:
Cardiac Magnetic Resonance Imaging vs Positron Emission Tomography in the Assessment of Viability in Ischemic Cardiomyopathy. The Alternative Imaging Modalities in Heart Failure (AIM-HF) Clinical Trial
Tavoosi Anahita, Garrard Linda, Kandolin Riina, Guo Ann, Dekemp Robert, Wells George, Beanlands Rob, Mielniczuk Lisa, Paterson David, Omeara Eileen, White James, Larose Eric, Knuuti Juhani, Wiefels Christiane, Chow Benjamin, Chen Li
A Growing Burden of Electronic Medical Record Messages in ACHD CareDailey Schwartz Andrew, Alegria Jorge