Final ID: 4169341
Myeloperoxidase Inhibition with Mitiperstat in Heart Failure with Preserved and Mildly Reduced Ejection Fraction: Primary Results from the ENDEAVOR Randomized Clinical Trial
Abstract Body (Do not enter title and authors here): Hypothesis/Purpose: Myeloperoxidase (MPO), an inflammatory mediator released by neutrophils, has been associated with microvascular dysfunction, myocardial fibrosis, and cardiomyocyte dysfunction. We hypothesized that MPO may be a novel treatment target for HFpEF.
Study Design/Methods: Multicenter, randomized, placebo-controlled, double-blind trial of the MPO inhibitor mitiperstat vs. placebo (NCT04986202), conducted from 2021–2024 at 142 sites in 18 countries.
Sample Size: n=711 randomized: mitiperstat 2.5 mg (n=235), 5.0 mg (n=240), and placebo (n=236).
Population Studied: Symptomatic HF (NYHA class II-IV) with LVEF >40%; KCCQ-TSS ≤90 points, 6MWD 30–400 meters; ↑NTproBNP; and (1) structural heart disease, (2) ↑LV filling pressure, (3) significant diastolic dysfunction, or (4) recent HF hospitalization.
Intervention: 48-wk treatment with mitiperstat 2.5 mg, mitiperstat 5.0 mg, or placebo (randomized 1:1:1, stratified by baseline neutrophil count [≤4 vs. >4 K/uL]).
Primary End Points: Change in KCCQ Total Summary Score (KCCQ-TSS) and 6-minute walk distance (6MWD) from baseline to 16 wks. An ANCOVA model was used to estimate treatment effects of mitiperstat vs. placebo.
Secondary End Points: Changes from baseline in KCCQ-TSS and 6MWD (24 and 48 wks); biomarkers (NTproBNP, CRP, IL-6; 6, 24, and 48 wks); and echo parameters (LV global longitudinal strain, LVMI, and LAVI; 16 and 24 wks). A composite CV endpoint (HF hospitalization, MI, or death during 48 wks of treatment) was an exploratory endpoint.
Power Calculations: Sample size of 600 (n=200 per arm [n=400 pooled 2.5 and 5.0 mg mitiperstat and n=200 placebo]) provided 85% power at α=0.05 to detect a minimum between-group difference of 6.0 points for △KCCQ-TSS (assumed SD=20 points) and 21 m for △6MWD (assumed SD=70 m).
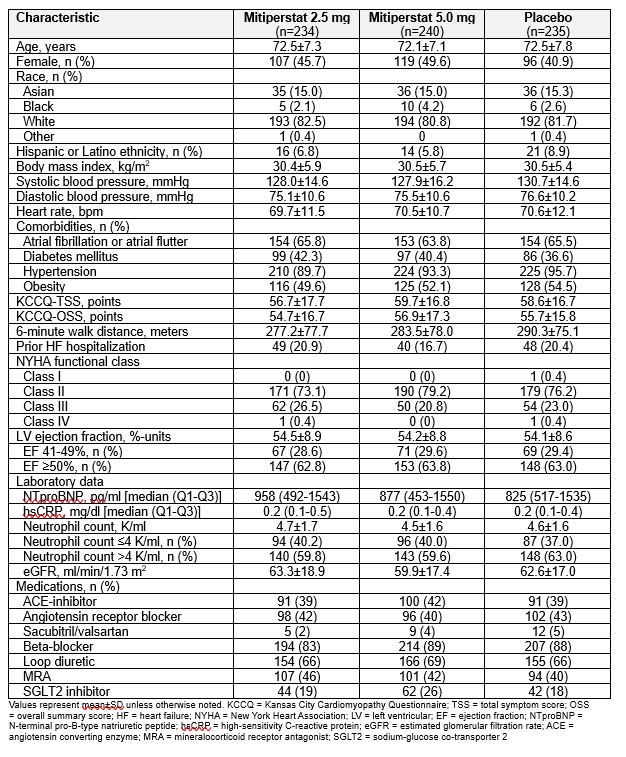
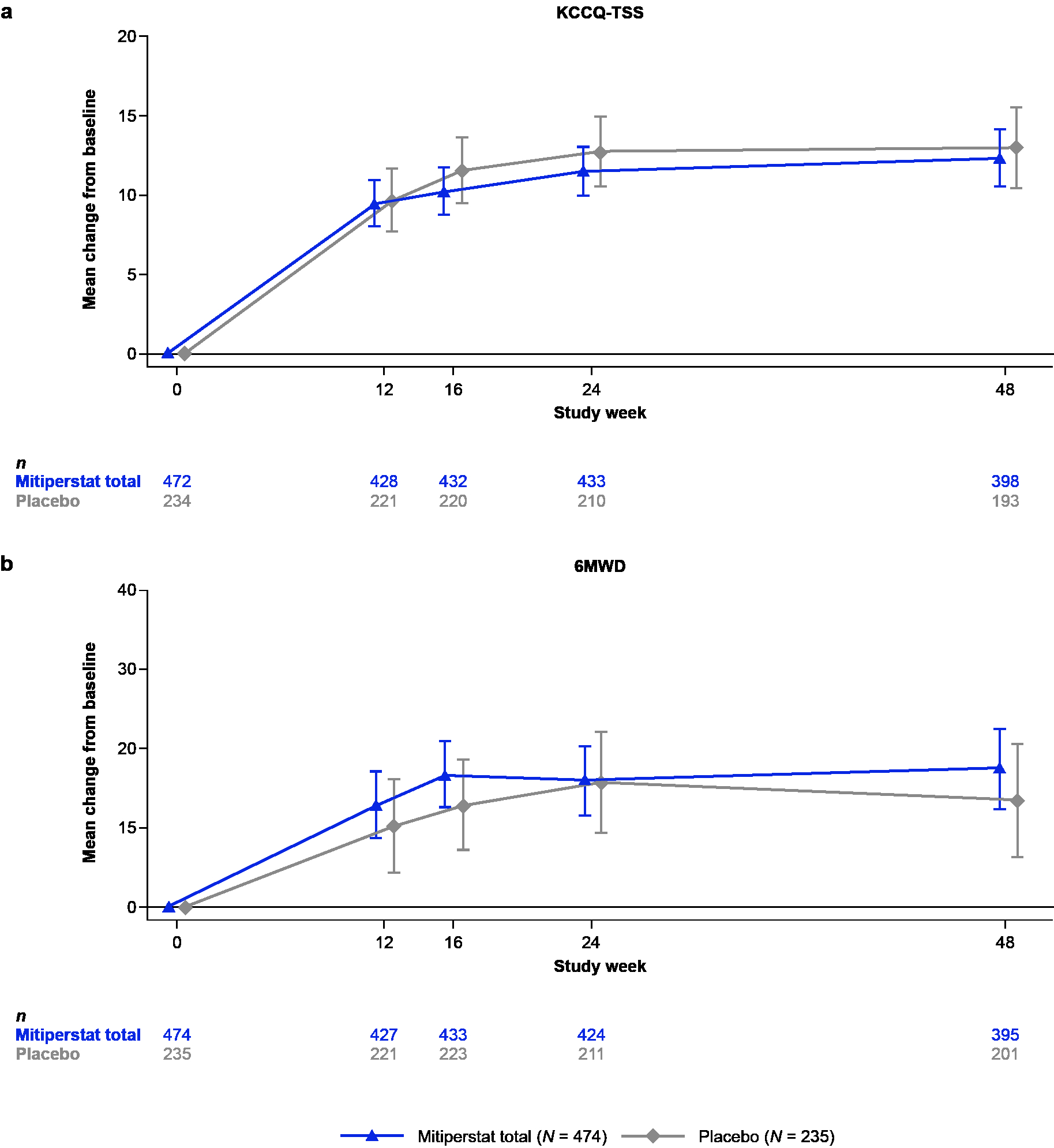
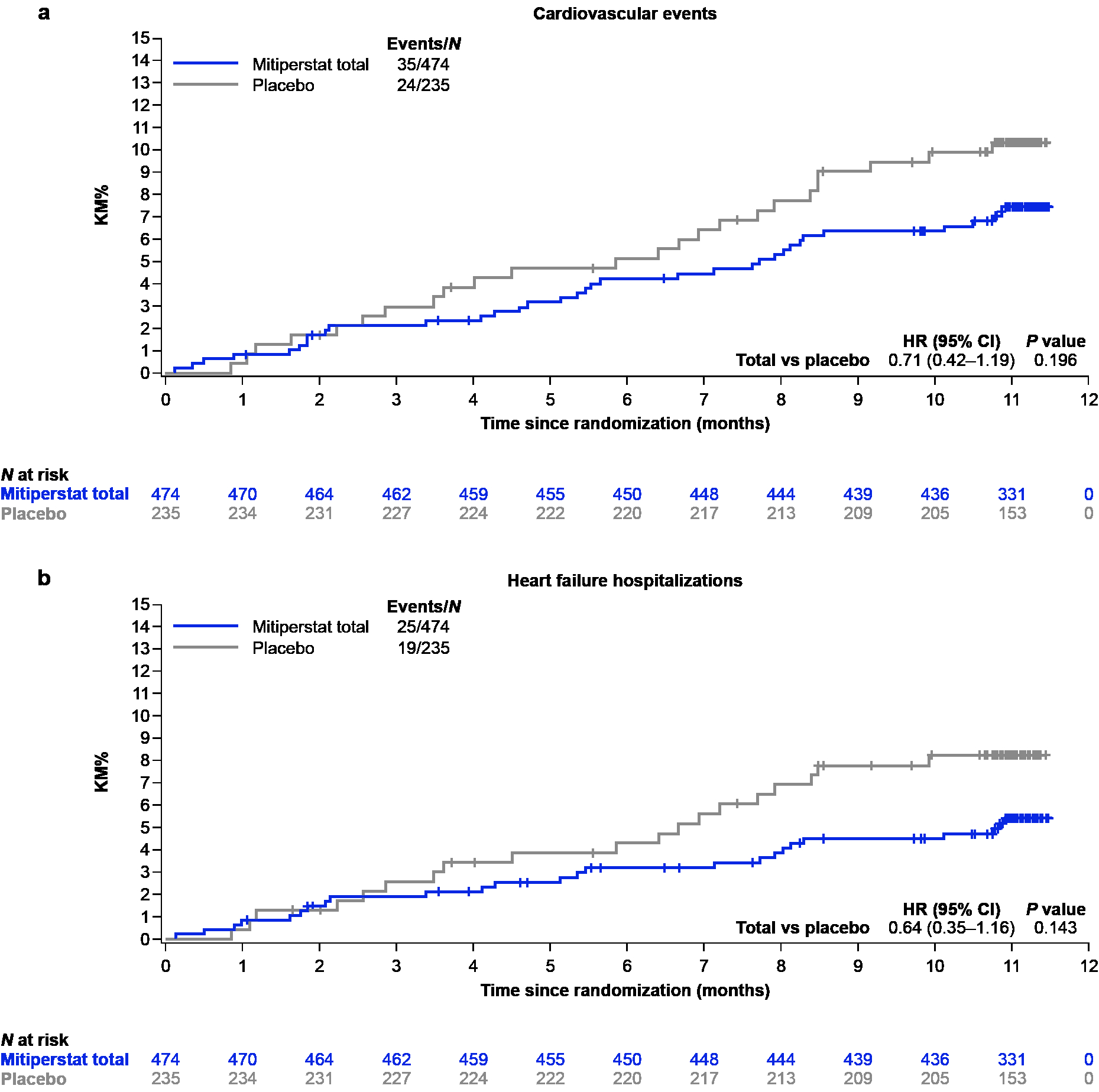
Results: Table 1 displays baseline characteristics. Mitiperstat (2.5 mg + 5.0 mg groups pooled) vs. placebo did not improve KCCQ-TSS or 6MWD (Fig. 1), or any of the secondary endpoints. Results were similar across pre-specified subgroups. Mitiperstat (vs. placebo) resulted in numerically fewer CV events (HR 0.71 [95% CI 0.42, 1.19]; P=0.20) and fewer HF hospitalizations (HR 0.64 [95% CI 0.35, 1.16]; P=0.14) (Fig. 2). AEs and SAEs were similar across groups.
Conclusions: Mitiperstat was safe but did not improve symptoms or exercise function over 16 weeks in HFpEF. The potential beneficial longer-term effect of mitiperstat on reducing HF hospitalization and death requires further investigation.
Study Design/Methods: Multicenter, randomized, placebo-controlled, double-blind trial of the MPO inhibitor mitiperstat vs. placebo (NCT04986202), conducted from 2021–2024 at 142 sites in 18 countries.
Sample Size: n=711 randomized: mitiperstat 2.5 mg (n=235), 5.0 mg (n=240), and placebo (n=236).
Population Studied: Symptomatic HF (NYHA class II-IV) with LVEF >40%; KCCQ-TSS ≤90 points, 6MWD 30–400 meters; ↑NTproBNP; and (1) structural heart disease, (2) ↑LV filling pressure, (3) significant diastolic dysfunction, or (4) recent HF hospitalization.
Intervention: 48-wk treatment with mitiperstat 2.5 mg, mitiperstat 5.0 mg, or placebo (randomized 1:1:1, stratified by baseline neutrophil count [≤4 vs. >4 K/uL]).
Primary End Points: Change in KCCQ Total Summary Score (KCCQ-TSS) and 6-minute walk distance (6MWD) from baseline to 16 wks. An ANCOVA model was used to estimate treatment effects of mitiperstat vs. placebo.
Secondary End Points: Changes from baseline in KCCQ-TSS and 6MWD (24 and 48 wks); biomarkers (NTproBNP, CRP, IL-6; 6, 24, and 48 wks); and echo parameters (LV global longitudinal strain, LVMI, and LAVI; 16 and 24 wks). A composite CV endpoint (HF hospitalization, MI, or death during 48 wks of treatment) was an exploratory endpoint.
Power Calculations: Sample size of 600 (n=200 per arm [n=400 pooled 2.5 and 5.0 mg mitiperstat and n=200 placebo]) provided 85% power at α=0.05 to detect a minimum between-group difference of 6.0 points for △KCCQ-TSS (assumed SD=20 points) and 21 m for △6MWD (assumed SD=70 m).
Results: Table 1 displays baseline characteristics. Mitiperstat (2.5 mg + 5.0 mg groups pooled) vs. placebo did not improve KCCQ-TSS or 6MWD (Fig. 1), or any of the secondary endpoints. Results were similar across pre-specified subgroups. Mitiperstat (vs. placebo) resulted in numerically fewer CV events (HR 0.71 [95% CI 0.42, 1.19]; P=0.20) and fewer HF hospitalizations (HR 0.64 [95% CI 0.35, 1.16]; P=0.14) (Fig. 2). AEs and SAEs were similar across groups.
Conclusions: Mitiperstat was safe but did not improve symptoms or exercise function over 16 weeks in HFpEF. The potential beneficial longer-term effect of mitiperstat on reducing HF hospitalization and death requires further investigation.
More abstracts on this topic:
Aberrant Trans- and De- Nitrosylation Underpins Nitrosative Stress in Cardiometabolic HFpEF
Li Zhen, Borch Jensen Martin, Vondriska Thomas, Lefer David, Gehred Natalie, Gromova Tatiana, Lapenna Kyle, Sharp Thomas, Chen Jingshu, Shambhu Smitha, Yu Xiaoman, Goodchild Traci
Antigen Presenting Cell-Specific Keap1-Nrf2 Pathway Mediates Salt-Sensitive HypertensionKhan Mohd, Saleem Mohammad, Kirabo Annet