Final ID: 4167869
Five-Year Results from the AMPLATZER Amulet Left Atrial Appendage Occluder Randomized Controlled Trial (Amulet IDE)
The Amulet IDE trial is a prospective, global, clinical trial with 1:1 randomization comparing the AMPLATZER™ Amulet™ Left Atrial Appendage (LAA) Occluder (Abbott) to the Watchman™ 2.5 LAA device (Boston Scientific) in patients with non-valvular atrial fibrillation. This is the first report of the 5-year results from the trial.
Methods
Patients enrolled were at a high risk of stroke or systemic embolism (SE) defined as CHA2DS2-VASc score of ≥3. Clinical follow-up occurred at discharge, 45 days, 3, 6, 9, 12, and 18 months and annually through 5 years. An independent echocardiographic core laboratory was used to analyze cardiac images and adverse events were adjudicated by an independent clinical events committee.
Results
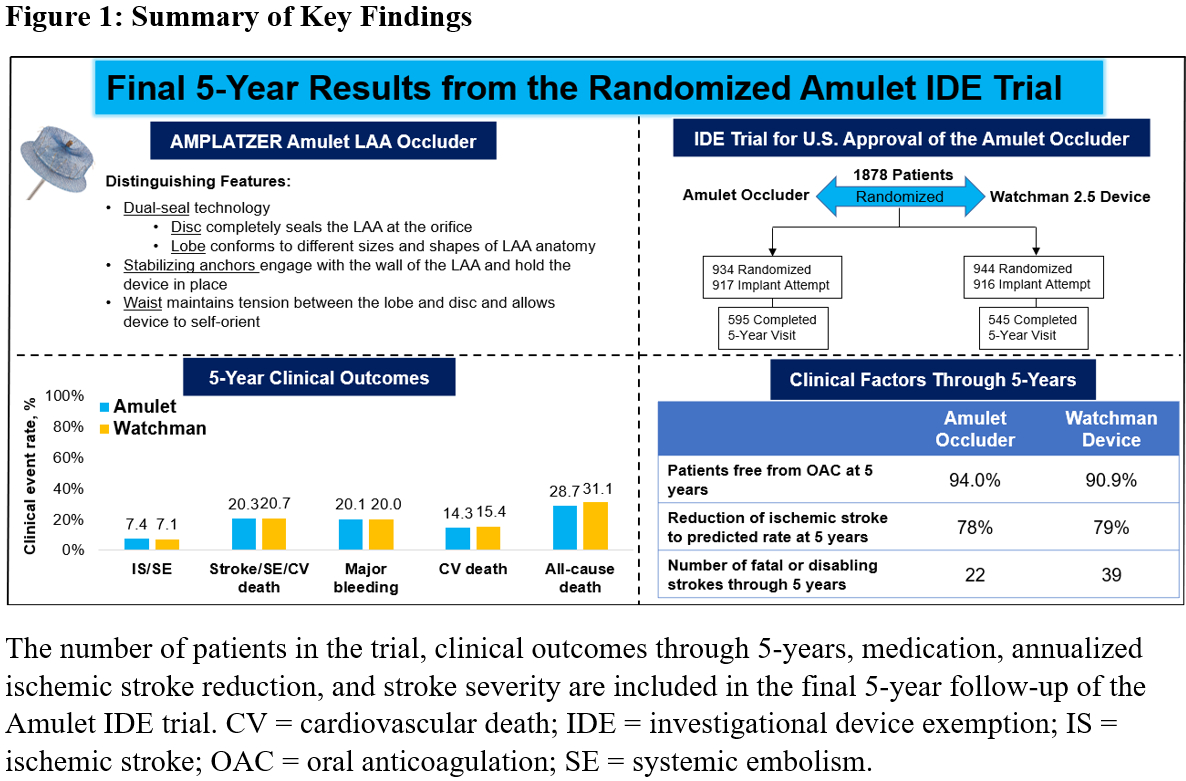
A total of 1,878 patients at 108 sites were randomized and 1,833 underwent an implant attempt (n=917 Amulet occluder and n=916 Watchman device) which were used in this analysis (Figure 1). The final 5-year follow-up visit rate was over 80% in both device groups (89.2%; N=595 in Amulet occluder and 83.3%; N=545 in Watchman device). Baseline characteristics were well-matched between the two device groups (75.1 years, 40.0% female, CHA2DS2-VASc: 4.6 and HAS-BLED: 3.3). At 5 years, 94.0% of patients were free of OAC in Amulet occluder patients compared to 90.9% in Watchman device patients (p=0.009). Five-year clinical outcomes were similar between the Amulet and Watchman device including the composite of ischemic stroke or SE (7.4% vs. 7.1%; p=0.851), composite of stroke, SE, or cardiovascular (CV) death (20.3% vs. 20.7%; p=0.666), major bleeding (20.1% vs. 20.0%; p=0.882), CV death (14.3% vs. 15.4%; p=0.429), and all-cause death (28.7% vs. 31.1%; p=0.217). Annualized ischemic stroke rates at 5 years were low and similar for Amulet (1.6%/year) and Watchman (1.6%/year) devices resulting in a 78% and 79% reduction in the risk of stroke based on the CHA2DS2-VASc scores. Strokes in patients with the Amulet occluder tended to be less severe (n=38 non-disabling, n=11 disabling, n=11 fatal, n=12 unknown) than strokes in patients with the Watchman device (n=19 non-disabling, n=22 disabling, n=17 fatal, n=10 unknown).
Conclusions
The 5-year outcomes from the largest randomized LAAO clinical trial demonstrated the long-term safety and effectiveness of the Amulet occluder and Watchman device. The dual-seal Amulet occluder reduces atrial fibrillation-related thromboembolic events while eliminating the need for long-term OAC.
- Lakkireddy, Dhanunjaya ( Kansas City Heart Rhythm Institute and Research Foundation , Overland Park , Kansas , United States )
- Schmidt, Boris ( CCB , Frankfurt , Germany )
- Horton, Rodney ( Texas Cardiac Arrhythmia Research F , Austin , Texas , United States )
- Gupta, Nigel ( KP Los Angeles Medical Center , Los Angeles , California , United States )
- Alkhouli, Mohamad Adnan ( Mayo Clinic , Rochester , Minnesota , United States )
- Windecker, Stephan ( Swiss Cardiovascular Center Bern , Bern , Switzerland )
- Ellis, Christopher ( Vanderbilt University Heart-EP , Nashville , Tennessee , United States )
- Thaler, David ( TUFTS MEDICAL CENTER , Boston , Massachusetts , United States )
- Swarup, Vijendra ( Arizona Cardiovascular Research Center , Phoenix , Arizona , United States )
- Gambhir, Alok ( Northside Hospital , Atlanta , Georgia , United States )
- Hermlller, James ( St. Vincent Medical Group, Inc. , Indianapolis , Indiana , United States )
- Nielsen-kudsk, Jens Erik ( Aarhus University Hospital , Aarhus N , Denmark )
- Worthley, Stephen ( Macquarie University Hospital , Macquarie Park , Australian Capital Territory , Australia )
- Nair, Devi ( St. Bernards Medical Center , Jonesboro , Arkansas , United States )
Meeting Info:
Session Info:
Featured Science: Novel Insights in Cardiovascular Interventional Outcomes
Monday, 11/18/2024 , 01:30PM - 02:45PM
Featured Science
More abstracts on this topic:
Callegari Santiago, Romain Gaelle, Balasquide-odeh Odaly, Tapia Christiany, Pinto Daniel, Rahman Mufti, Vashist Aseem, Smolderen Kim, Mena-hurtado Carlos
A-band titin-truncating variant promotes the development of arrhythmia-induced cardiomyopathy in a novel genetically-engineered porcine modelLee Kwonjae, Del Rio Carlos, Mcnally Elizabeth, Pfenniger Anna, Bhatnagar Ashita, Glinton Kristofor, Burrell Amy, Ober Rebecca, Mcluckie Alicia, Bishop Brian, Rogers Christopher, Geist Gail
More abstracts from these authors:
Windecker Stephan, Steg Philippe
New technologies and indications for left atrial appendage occlusionNair Devi, Alkhouli Mohamad, Tamis-holland Jacqueline, Ellis Christopher