Final ID: 4161507
Bempedoic Acid and Limb Outcomes in Statin-Intolerant Patients with Peripheral Artery Disease: New insights from the Clear Outcomes Trial
Abstract Body (Do not enter title and authors here): Study Design and Methods:
CLEAR Outcomes randomized 13,970 patients to bempedoic acid 180 mg or placebo. A clinical history of PAD was reported by investigators at baseline. Two blinded vascular medicine specialists independently adjudicated all adverse events for limb outcomes including worsening PAD symptoms leading to revascularization, chronic limb threatening ischemia, and acute limb ischemia events with the composite defined as MALE. Outcomes were assessed as time to first event as well as total (including recurrent) events using a negative binomial approach.
Sample Size: 13,970 (including 1,624 with PAD)
Population Studied: Statin intolerant patients – primary and secondary prevention
Intervention(s): Bempedoic acid
Power Calculations: Primary trial was designed to accrue 1620 events and provide ≥90% power to detect a 15% reduction in MACE-4 with two-sided significance level of 0.05. The current analysis includes over 3000 MACE+MALE events including >400 confirmed limb outcomes.
Primary End Points: Major adverse limb events (MALE) including worsening symptoms leading to revascularization, chronic limb threatening ischemia, and acute limb ischemia.
Secondary End Points: Composite of MACE and MALE, total (including recurrent) MACE and MALE events.
Outcome(s)[Statistical Plan or Main Results
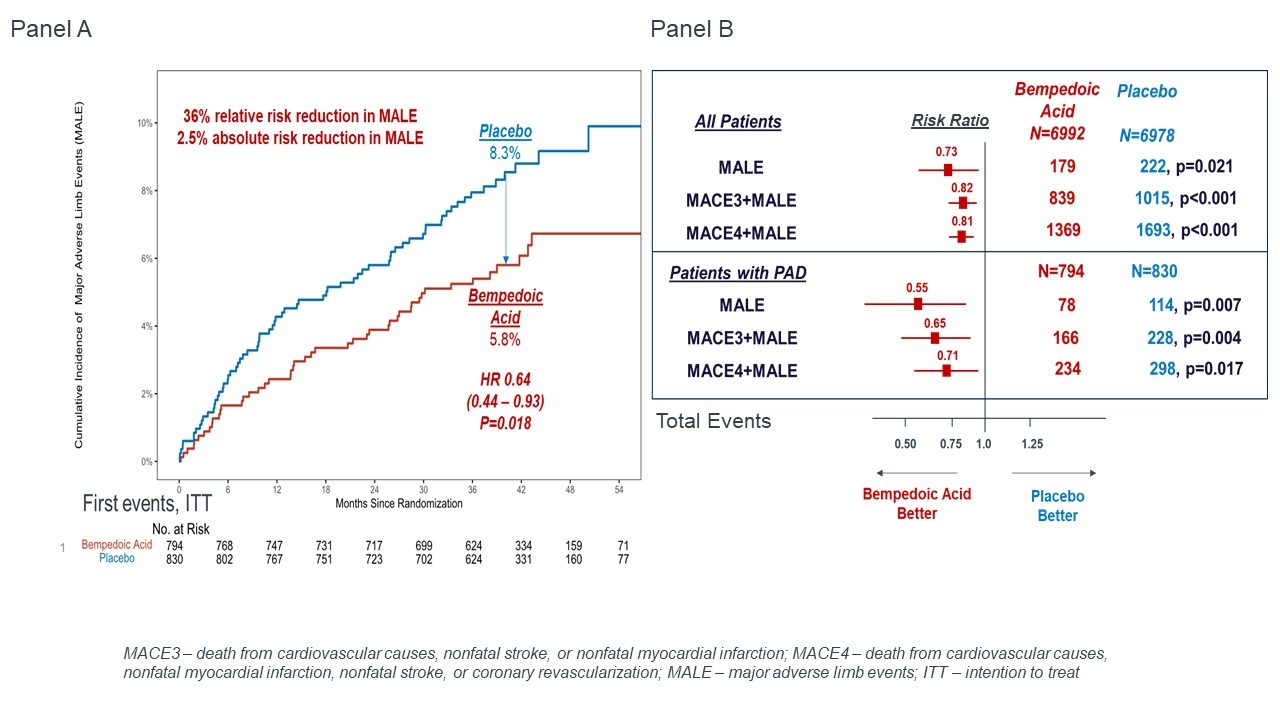
A total of 1,624 of the enrolled patients had PAD at baseline. Over a median of 41.1 months the rate of first and total MALE events in patients with PAD in the placebo group were 8.3% and 13.7% respectively. Bempedoic acid reduced first MALE events by 36% (HR 0.64, 95% CI 0.44-0.93) with an absolute risk reduction (ARR) of 2.5% and number needed to treat (NNT) of 40, compared to placebo (figure, panel A). Similarly, when considering total events, bempedoic acid reduced MALE by 45% (RR 0.55, 95% CI 0.35 – 0.85, figure panel b). Consistent benefits were observed for MALE in the overall population as well as composites of MACE and MALE in both populations (Figure panel b). There was no evidence of effect modification for the benefit of bempedoic acid for MALE on the basis of PAD at baseline (p-interaction 0.09).
Bempedoic acid significantly reduces MALE. Benefits appear potentially greater when considering total (i.e. recurrent) events. These findings support the importance of LDL-C lowering therapy in patients with PAD. In addition, these findings support the early use of therapies with proven MALE benefit, including bempedoic acid, to optimize outcomes in PAD.
CLEAR Outcomes randomized 13,970 patients to bempedoic acid 180 mg or placebo. A clinical history of PAD was reported by investigators at baseline. Two blinded vascular medicine specialists independently adjudicated all adverse events for limb outcomes including worsening PAD symptoms leading to revascularization, chronic limb threatening ischemia, and acute limb ischemia events with the composite defined as MALE. Outcomes were assessed as time to first event as well as total (including recurrent) events using a negative binomial approach.
Sample Size: 13,970 (including 1,624 with PAD)
Population Studied: Statin intolerant patients – primary and secondary prevention
Intervention(s): Bempedoic acid
Power Calculations: Primary trial was designed to accrue 1620 events and provide ≥90% power to detect a 15% reduction in MACE-4 with two-sided significance level of 0.05. The current analysis includes over 3000 MACE+MALE events including >400 confirmed limb outcomes.
Primary End Points: Major adverse limb events (MALE) including worsening symptoms leading to revascularization, chronic limb threatening ischemia, and acute limb ischemia.
Secondary End Points: Composite of MACE and MALE, total (including recurrent) MACE and MALE events.
Outcome(s)[Statistical Plan or Main Results
A total of 1,624 of the enrolled patients had PAD at baseline. Over a median of 41.1 months the rate of first and total MALE events in patients with PAD in the placebo group were 8.3% and 13.7% respectively. Bempedoic acid reduced first MALE events by 36% (HR 0.64, 95% CI 0.44-0.93) with an absolute risk reduction (ARR) of 2.5% and number needed to treat (NNT) of 40, compared to placebo (figure, panel A). Similarly, when considering total events, bempedoic acid reduced MALE by 45% (RR 0.55, 95% CI 0.35 – 0.85, figure panel b). Consistent benefits were observed for MALE in the overall population as well as composites of MACE and MALE in both populations (Figure panel b). There was no evidence of effect modification for the benefit of bempedoic acid for MALE on the basis of PAD at baseline (p-interaction 0.09).
Bempedoic acid significantly reduces MALE. Benefits appear potentially greater when considering total (i.e. recurrent) events. These findings support the importance of LDL-C lowering therapy in patients with PAD. In addition, these findings support the early use of therapies with proven MALE benefit, including bempedoic acid, to optimize outcomes in PAD.
More abstracts on this topic:
A systematic review and meta-analysis comparing the effect of remote ischemic preconditioning on patients with intermittent claudication
Pinheiro Bruno, Pille Julia
A Genome-wide CRISPRi Screen Implicates Coronary Artery Disease GWAS Genes as Key Regulators of Adventitial Fibroblast ProliferationJackson William, Zhu Ashley, Gu Wenduo, Berezowitz Alexa, Iyer Meghana, Cheng Paul