Final ID: 4159375
Catheter Ablation or Antiarrhythmic Drugs for Ventricular Tachycardia in Ischemic Cardiomyopathy
Hypothesis and Purpose: The VANISH2 trial tests the hypothesis that catheter ablation (CA) is superior to antiarrhythmic drug (AAD) therapy for reducing incidence of the composite endpoint of death, VT storm, appropriate implantable defibrillator (ICD) shock, or sustained VT below ICD detection.
Study Design and Methods: In this multicenter parallel-group two-arm prospective open-label randomized clinical trial with blinded adjudication of endpoints patients were randomly allocated to CA or AAD therapy.
Sample Size: 416
Population Studied: Patients with prior myocardial infarction and a qualifying VT event consisting of at least one of: sustained monomorphic VT terminated by intervention; ≥3 episodes of VT treated by antitachycardia pacing (ATP) by an ICD with symptoms; ≥5 episodes of VT treated by ATP regardless of symptoms; ≥1 appropriate ICD shock; or ≥3 episodes of VT in a 24 hour period. Qualifying events occurred while patients were not treated with AADs.
Interventions: Participants were randomized to receive either CA or to be treated with AADs. Based upon pre-specified clinical criteria, participants were classified as either eligible for sotalol or for amiodarone; randomization was stratified by drug eligibility. CA followed a standard protocol including ablation of ventricular substrate to suppress all inducible VT. Sotalol-eligible patients randomized to AAD received 120 mg twice daily; amiodarone-eligible patients randomized to AAD received standardized loading over 6 weeks then 200 mg daily. ICD programming was standardized.
Power Calculations: 416 patients were included to achieve 85% power to detect (with significance 0.05) a 35% reduction in the primary endpoint.
Primary End Points: The primary endpoint is a composite of death at any time, and, after 14 days, appropriate ICD shock, VT storm (≥3 VT events within 24 hours), or treated sustained VT below the detection interval of the ICD.
Secondary End Points: Secondary endpoints include the individual components of the primary endpoint, and several further prespecified clinical, arrhythmia and safety outcomes.
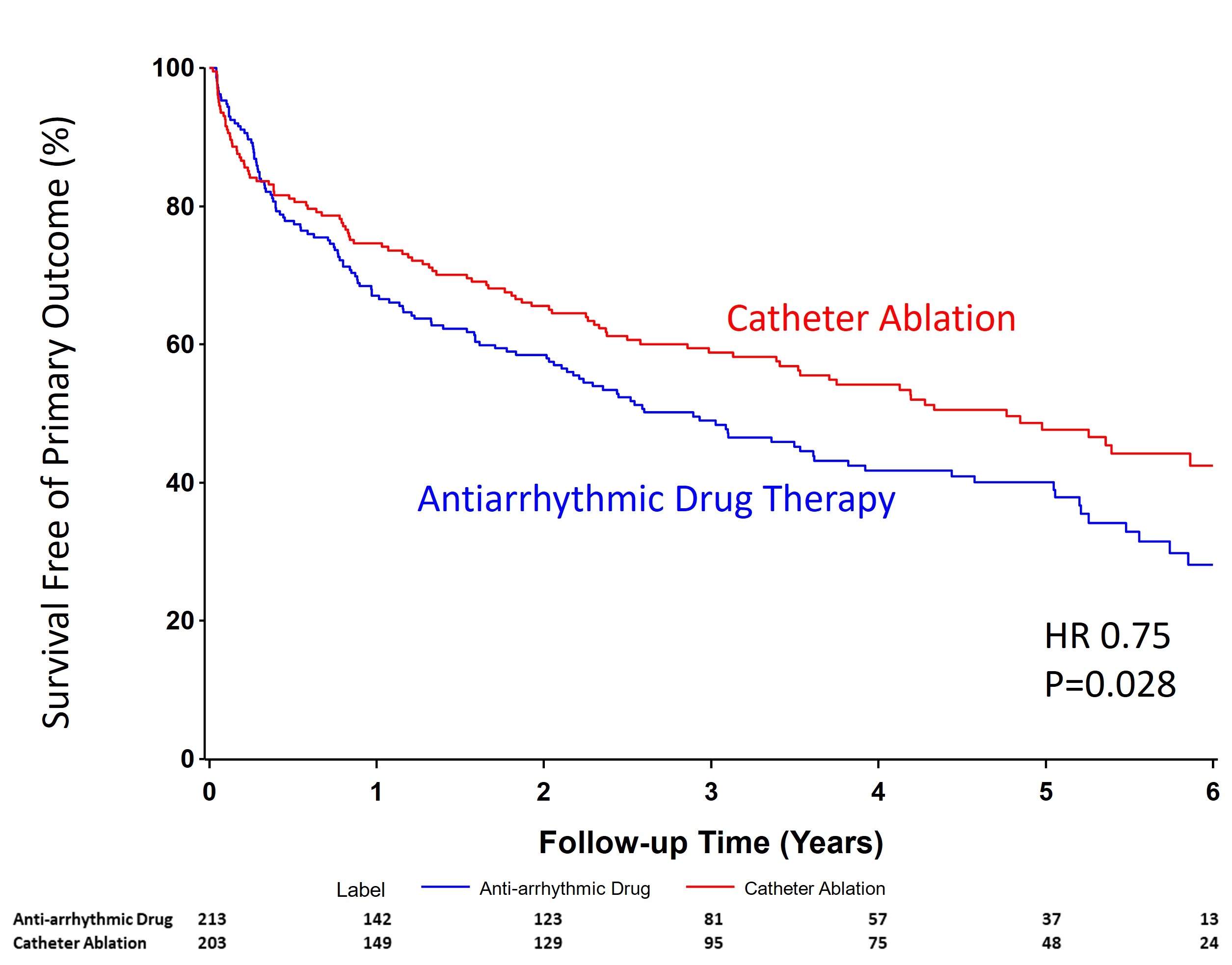
Outcomes: Statistical methods have been published (Sapp et al, Am Heart J. 2024;274;1-10). The rate of the primary endpoint was significantly lower in the ablation group than in the antiarrhythmic drug therapy group (HR 0.75; 95% CI 0.58,0.97; P=0.028). Full results will be presented.
- Sapp, John ( QEII HEALTH SCIENCES CENTRE , Halifax , Nova Scotia , Canada )
- Roux, Jean-francois ( University of Sherbrooke , Sherbrooke , Quebec , Canada )
- Nery, Pablo ( Ottawa Heart Institute , Ottawa , Ontario , Canada )
- Nault, Isabelle ( Institut Universitaire de Cardiologie et de Pneumology de Quebec , Quebec , Quebec , Canada )
- Amit, Guy ( Hamilton Health Sciences Centre , Hamilton , Ontario , Canada )
- Raymond, Jean-marc ( Centre Hospitalier de L'Universite de Montreal , Montreal , Quebec , Canada )
- Deyell, Marc ( University of British Columbia , Vancouver , British Columbia , Canada )
- Sacher, Frederic ( IHU LIRYC, Bordeaux University , Bordeaux , France )
- De Chillou, Christian ( Centre Hospitalier Universitaire de Nancy , Nancy , France )
- Kuriachan, Vikas ( University of Calgary , Calgary , Alberta , Canada )
- Abdelwahab, Amir ( QE II Health Sciences Centre , Halifax , Nova Scotia , Canada )
- Tang, Anthony ( Western University , London , Ontario , Canada )
- Sarrazin, Jean-francois ( Institut Universitaire de Cardiologie et de Pneumology de Quebec , Quebec , Quebec , Canada )
- Dyrda, Katia ( Montreal Heart Institute , Montreal , Quebec , Canada )
- Sikkel, Markus ( Western Cardiology Assoc and RJH , Victoria , British Columbia , Canada )
- Wilton, Stephen ( University of Calgary , Calgary , Alberta , Canada )
- Jolly, Umjeet ( St. Mary's General Hospital , Kitchener , Ontario , Canada )
- Kanagasundram, Arvindh ( VANDERBILT , Brentwood , Tennessee , United States )
- Wells, George ( University of Ottawa, Heart Inst. , Ottawa , Ontario , Canada )
- Parkash, Ratika ( QEII HEALTH SCIENCES CENTER , Halifax , Nova Scotia , Canada )
- Stevenson, William ( Vanderbilt University Medical Ctr , Nashville , Tennessee , United States )
- Healey, Jeff ( McMaster University , Hamilton , Ontario , Canada )
- Gula, Lorne ( UNIV OF WESTERN ONTARIO , London , Ontario , Canada )
- Nair, Girish ( University of Ottawa Heart Institut , Ottawa , Ontario , Canada )
- Essebag, Vidal ( McGill University Health Centre , Montreal , Quebec , Canada )
- Rivard, Lena ( Montreal Heart Institute , Montreal , Quebec , Canada )
Meeting Info:
Session Info:
Redefining Arrhythmia Treatment: Pushing Boundaries
Saturday, 11/16/2024 , 01:30PM - 02:45PM
Late-Breaking Science
More abstracts on this topic:
Wexler Yehuda, Grinstein Harel, Landesberg Michal, Glatstein Shany, Huber Irit, Arbel Gil, Gepstein Lior
A novel risk score predicts the prevalence of left atrial low-voltage areas and rhythm outcome in patients undergoing long-standing persistent atrial fibrillation ablationOoka Hirotaka, Nakao Sho, Kusuda Masaya, Ariyasu Wataru, Kudo Satoshi, Fujii Subaru, Mano Toshiaki, Matsuda Yasuhiro, Masuda Masaharu, Okamoto Shin, Ishihara Takayuki, Nanto Kiyonori, Tsujimura Takuya, Hata Yosuke, Uematsu Hiroyuki
More abstracts from these authors:
Dai Yuchen, Wells George, Beanlands Rob, Birnie David, Dekemp Robert, Nery Pablo, Tavoosi Anahita, Nair Girish, Redpath Calum, Golian Mehrdad, Thornhill Rebecca, Pena-fernandez Elena, Hansom Simon, Sadek Mouhannad
The Need for Ongoing Oral Anticoagulation in Patients with Clinical Stroke Risk Factors After Successful Catheter Ablation of Atrial Fibrillation: The OCEAN Randomized TrialVerma Atul, Essebag Vidal, Champagne Jean, Hill Michael, Smith Eric, Wells George, Birnie David, Jiang Chenyang, Heidbuchel Hein, Hindricks Gerhard, Kirchhof Paulus, Healey Jeff, Sharma Mike, Ha Andrew