Final ID: 4146689
Mavacamten: Real-World Experience from 22 Months of the Risk Evaluation and Mitigation Strategy (REMS) Program
Abstract Body (Do not enter title and authors here): Introduction
Mavacamten is a cardiac myosin inhibitor approved by the US FDA for the treatment of adults with symptomatic New York Heart Association class II-III obstructive hypertrophic cardiomyopathy to improve functional capacity and symptoms. Under the risk evaluation and mitigation strategy (REMS) program, patients taking mavacamten are required to be monitored for development of systolic heart failure (HF), with interruption if left ventricular ejection fraction (LVEF) decreases to <50%.
Aims
We report updated results from the mavacamten REMS database (28-Apr-2022 to 27-Feb-2024), including safety data for patients treated for ≥1 year.
Methods
Data on health care providers and pharmacy certification, patient monitoring (including health status forms and echocardiograms), and screening for drug interactions prior to each dispense were collected.
Results
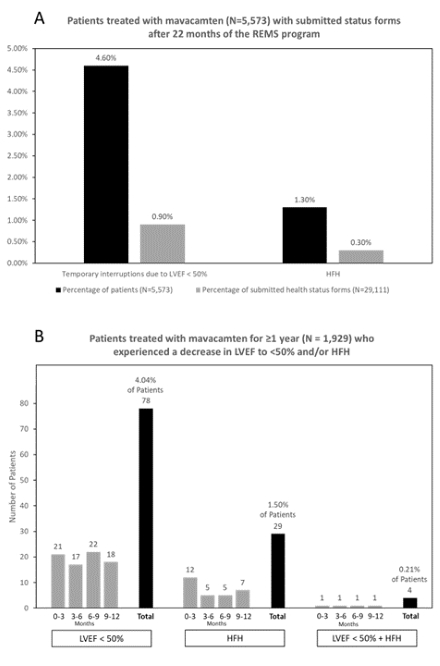
Of 6,299 patients who received ≥1 dose of mavacamten, 60.0% were women; ages were <18 (0.1%), 18-40 (6.3%), 41-60 (29.0%), and ≥61 years (64.6%). Of 5,573 patients with submitted health status forms as of 27-Feb-2024, 256 (4.6%; Figure A) experienced a decrease in LVEF to <50% and 71 (1.3%) experienced HF requiring hospitalization (HFH). On the 29,111 status forms, LVEF <50% was reported on 276 (0.9%) and HFH was reported on 86 (0.3%). Of 1,929 patients with ≥1 year of treatment (Figure B), 78 (4.04%) had an LVEF reduction to <50% and 4 (0.21%) experienced LVEF <50% + HFH (with potential confounders like atrial fibrillation) but later resumed treatment. A planned dosing analysis will provide further insights. Of 52,432 drug interaction checklists completed, 130 (0.25%) resulted in mavacamten dose reduction and 167 (0.32%) resulted in discontinuation of a concurrent medication.
Conclusions
In >6,000 patients during the first 22 months of the REMS Program for mavacamten, the need for temporary interruption for LVEF <50% was very low, including for patients on therapy ≥1 year, with even fewer LVEF reductions associated with HFH. Additionally, a low proportion of patients had potential drug-drug interactions, with a low number requiring a mavacamten dose reduction. The data suggest favorable real-world experience with mavacamten, confirmed by REMS oversight.
Mavacamten is a cardiac myosin inhibitor approved by the US FDA for the treatment of adults with symptomatic New York Heart Association class II-III obstructive hypertrophic cardiomyopathy to improve functional capacity and symptoms. Under the risk evaluation and mitigation strategy (REMS) program, patients taking mavacamten are required to be monitored for development of systolic heart failure (HF), with interruption if left ventricular ejection fraction (LVEF) decreases to <50%.
Aims
We report updated results from the mavacamten REMS database (28-Apr-2022 to 27-Feb-2024), including safety data for patients treated for ≥1 year.
Methods
Data on health care providers and pharmacy certification, patient monitoring (including health status forms and echocardiograms), and screening for drug interactions prior to each dispense were collected.
Results
Of 6,299 patients who received ≥1 dose of mavacamten, 60.0% were women; ages were <18 (0.1%), 18-40 (6.3%), 41-60 (29.0%), and ≥61 years (64.6%). Of 5,573 patients with submitted health status forms as of 27-Feb-2024, 256 (4.6%; Figure A) experienced a decrease in LVEF to <50% and 71 (1.3%) experienced HF requiring hospitalization (HFH). On the 29,111 status forms, LVEF <50% was reported on 276 (0.9%) and HFH was reported on 86 (0.3%). Of 1,929 patients with ≥1 year of treatment (Figure B), 78 (4.04%) had an LVEF reduction to <50% and 4 (0.21%) experienced LVEF <50% + HFH (with potential confounders like atrial fibrillation) but later resumed treatment. A planned dosing analysis will provide further insights. Of 52,432 drug interaction checklists completed, 130 (0.25%) resulted in mavacamten dose reduction and 167 (0.32%) resulted in discontinuation of a concurrent medication.
Conclusions
In >6,000 patients during the first 22 months of the REMS Program for mavacamten, the need for temporary interruption for LVEF <50% was very low, including for patients on therapy ≥1 year, with even fewer LVEF reductions associated with HFH. Additionally, a low proportion of patients had potential drug-drug interactions, with a low number requiring a mavacamten dose reduction. The data suggest favorable real-world experience with mavacamten, confirmed by REMS oversight.
More abstracts on this topic:
4-Hydroxy-2-Nonenal Alters Alternative Polyadenylation to Regulate mRNA Isoform Diversity in the Transition from Human Cardiac Fibroblasts to Myofibroblasts
Natarajan Kartiga, Neupane Rahul, Yalamanchili Hari Krishna, Palaniyandi Suresh, Wagner Eric, Guha Ashrith, Amirthalingam Thandavarayan Rajarajan
A Case Presentation of Severe Left Ventricular Dysfunction from Focal Myocarditis due to Immune Checkpoint InhibitorPatel Romil, Hussain Kifah, Gordon Robert