Final ID: MDP416
Deep Learning-enabled Detection of Aortic Stenosis from Noisy Single-lead Electrocardiograms
Abstract Body (Do not enter title and authors here): BACKGROUND: Despite vast improvements in management strategies, aortic stenosis (AS) remains underdiagnosed, posing a substantial healthcare burden. Deep learning algorithms applied to portable ECGs can enable community screening for AS in older adults. However, noise in portable ECGs often hinders model performance. To address this, we developed and validated a noise-adapted AI algorithm to detect AS from 1-lead ECG and compared it against 12-lead ECGs in a large diverse US health system.
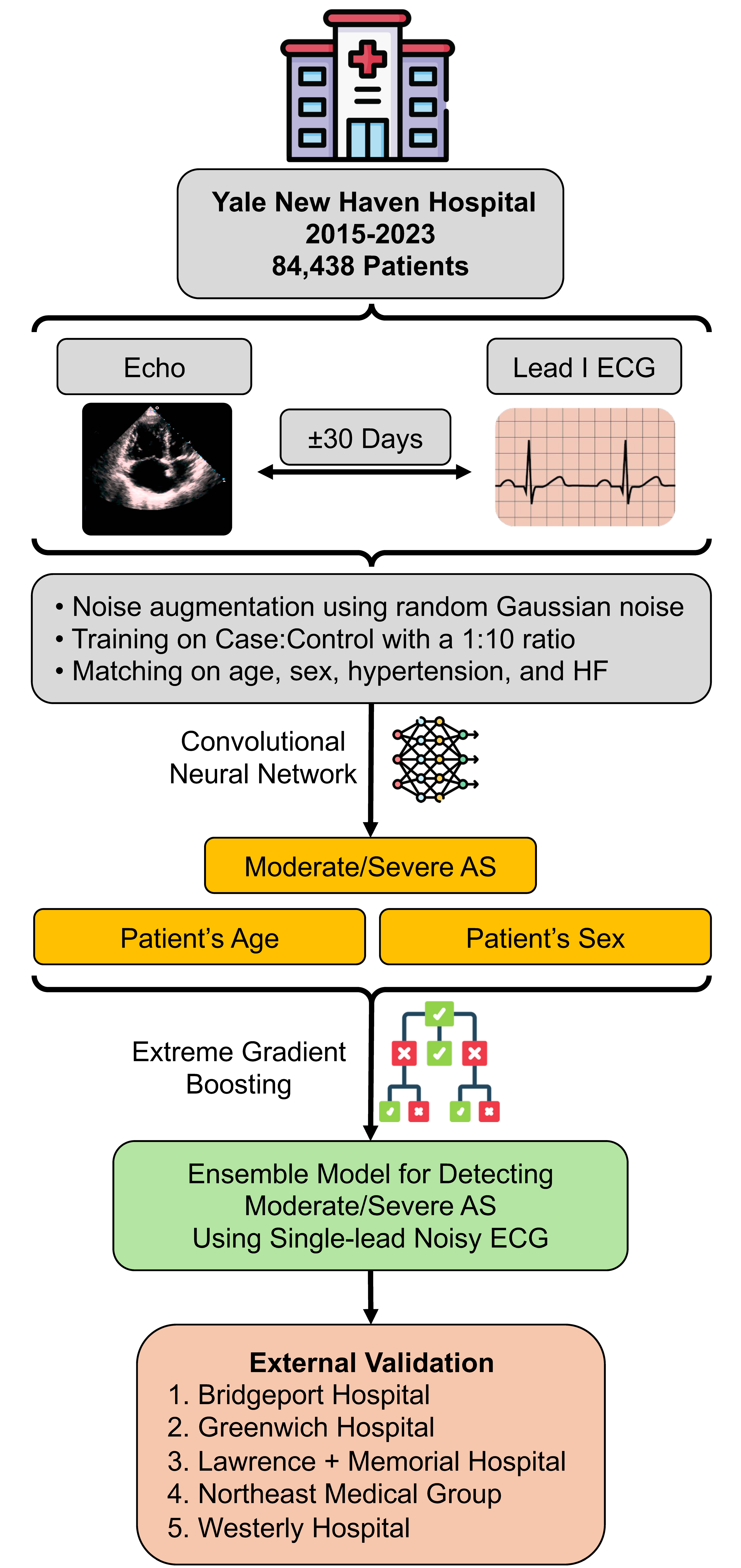
METHODS: Using lead I ECG among individuals with echocardiograms within a 30-day window from 84,438 patients at Yale (2015-2023), we developed a 1-lead AS screening model to detect moderate/severe AS. We validated this in 65,988 patients from 5 geographically distinct affiliated community hospitals and practices. Random Gaussian noise was introduced during training to improve the model’s resilience to noisy acquisition. To enforce learning AS-specific signatures as opposed to confounders, each 1-lead ECG of a patient with AS was matched with 10 controls on age, sex, hypertension, and heart failure. We trained an ensemble deep learning model using 1-lead ECGs, and patients’ age and sex as inputs to detect AS. Using the same strategy, we evaluated the performance of models trained using 12-lead ECGs.
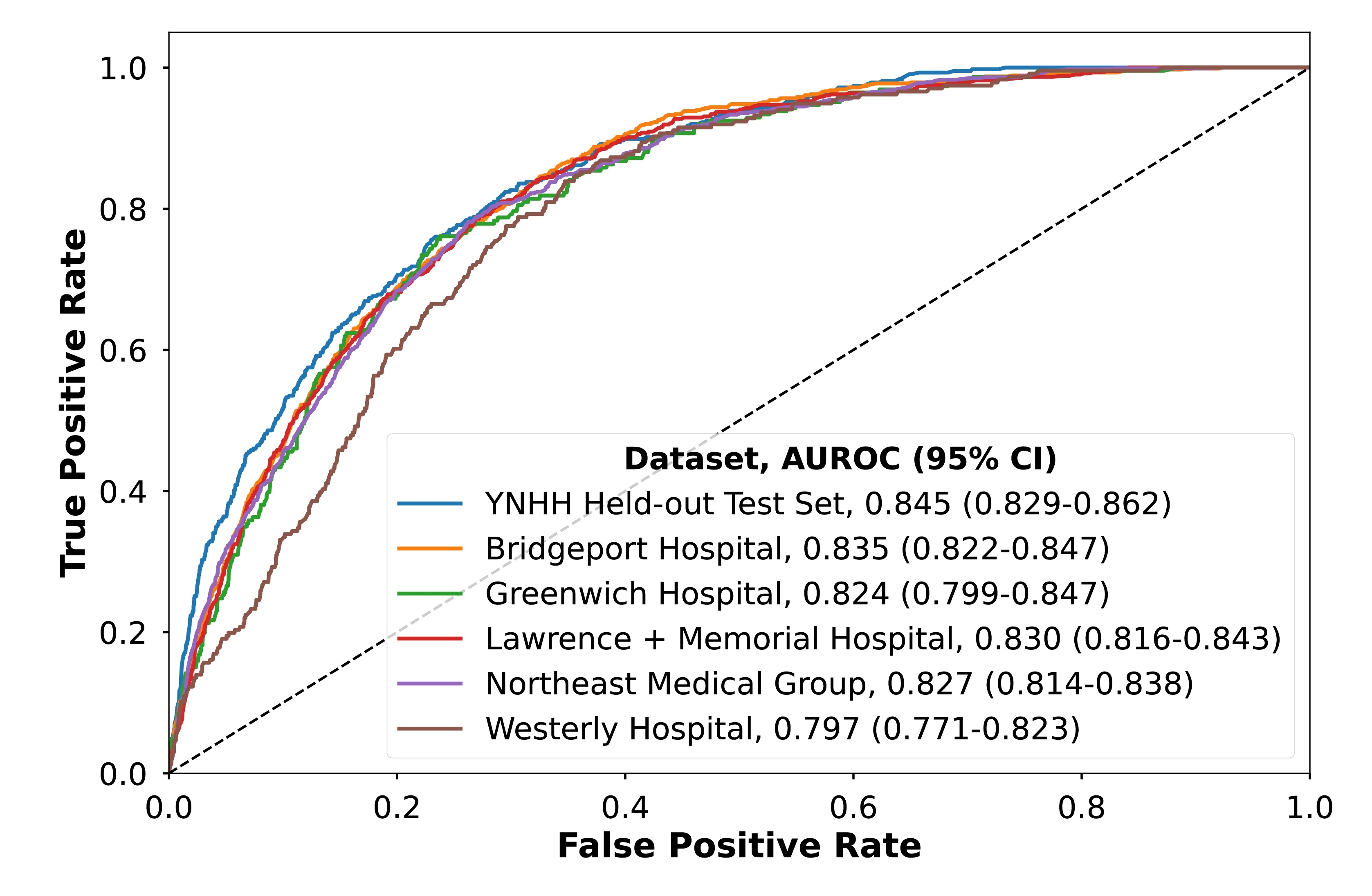
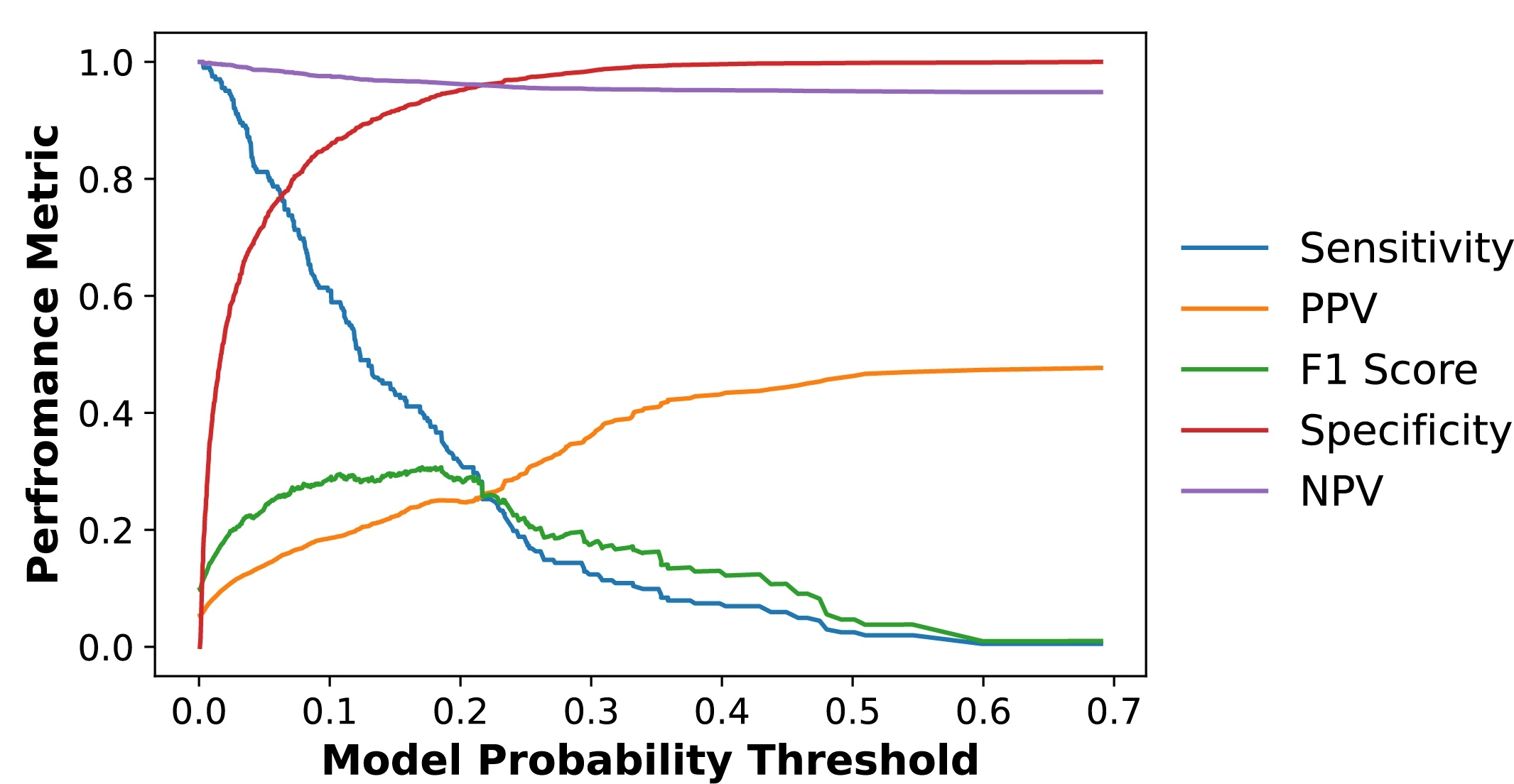
RESULTS: In the development set, 5.3% had AS compared with 4.5-9.3% across the validation sites. The ensemble 1-lead model achieved an AUROC of 0.845 (95% CI, 0.829-0.862) for detecting moderate or severe AS with consistent performance across community data sources (AUROC: 0.797-0.835). The discrimination of the 1-lead model was comparable to those achieved by 12-lead models (0.864 [0.848-0.879]). The model probability was progressively associated with a higher prevalence of AS (ORs for Q2 vs Q1, 35 [5-254]; Q3 vs Q1, 91 [13-654]; Q4 vs Q1, 388 [55-2,768]). The model output can be optimized to enable either a high sensitivity of >90% (with corresponding specificity of 63%, PPV of 12%, and NPV of 99%) or for specificity of >90% to reduce false positive results (with sensitivity 53%, PPV of 23%, and NPV of 97%).
CONCLUSION: A noise-adapted AI model can detect clinically significant AS from 1-lead ECGs, with performance similar to models trained on full 12-lead ECGs. Given its ease of deployment on wearable and portable devices, this represents a scalable screening strategy for asymptomatic AS in the community.
METHODS: Using lead I ECG among individuals with echocardiograms within a 30-day window from 84,438 patients at Yale (2015-2023), we developed a 1-lead AS screening model to detect moderate/severe AS. We validated this in 65,988 patients from 5 geographically distinct affiliated community hospitals and practices. Random Gaussian noise was introduced during training to improve the model’s resilience to noisy acquisition. To enforce learning AS-specific signatures as opposed to confounders, each 1-lead ECG of a patient with AS was matched with 10 controls on age, sex, hypertension, and heart failure. We trained an ensemble deep learning model using 1-lead ECGs, and patients’ age and sex as inputs to detect AS. Using the same strategy, we evaluated the performance of models trained using 12-lead ECGs.
RESULTS: In the development set, 5.3% had AS compared with 4.5-9.3% across the validation sites. The ensemble 1-lead model achieved an AUROC of 0.845 (95% CI, 0.829-0.862) for detecting moderate or severe AS with consistent performance across community data sources (AUROC: 0.797-0.835). The discrimination of the 1-lead model was comparable to those achieved by 12-lead models (0.864 [0.848-0.879]). The model probability was progressively associated with a higher prevalence of AS (ORs for Q2 vs Q1, 35 [5-254]; Q3 vs Q1, 91 [13-654]; Q4 vs Q1, 388 [55-2,768]). The model output can be optimized to enable either a high sensitivity of >90% (with corresponding specificity of 63%, PPV of 12%, and NPV of 99%) or for specificity of >90% to reduce false positive results (with sensitivity 53%, PPV of 23%, and NPV of 97%).
CONCLUSION: A noise-adapted AI model can detect clinically significant AS from 1-lead ECGs, with performance similar to models trained on full 12-lead ECGs. Given its ease of deployment on wearable and portable devices, this represents a scalable screening strategy for asymptomatic AS in the community.
More abstracts on this topic:
Advanced waveform analysis of the electrocardiogram signals using complementary signal processing techniques to investigate the response to a QTc prolonging drug vs control including food effect.
Serna Pascual Miquel, Nandi Manasi, Taubel Jorg, Lorch Ulrike, Djumanov Dilshat, Sadsad Tyrone-joshua, Mejia Elisa, Rickard James
A Machine Learning Approach to Predict Percutaneous Coronary Intervention in Patients with Critical Illness and Signs of Myocardial InjuryMueller Joshua, Stepanova Daria, Chidambaram Vignesh, Nakarmi Ukash, Al'aref Subhi