Final ID: 4146398
Single-Nucleus Transcriptome Profiling Identifies Phenotypic Shift in Pulmonary Vascular Endothelial Subpopulations in Pulmonary Arterial Hypertension
Abstract Body (Do not enter title and authors here): Rationale: Pulmonary arterial hypertension (PAH) is a devastating disease characterized by a progressive rise in pulmonary vascular resistance and occlusive vascular remodeling, leading to right heart failure and premature death. Endothelial cell (EC) damage and dysfunction is a key feature of pulmonary vascular remodeling and PAH. However, the understanding of how different EC subpopulations contribute to this process is limited.
Methods: We utilized single-nucleus RNA sequencing (snRNAseq) to provide transcriptome insights into cellular composition changes within idiopathic PAH (IPAH) and donor lungs. Principal component analysis (PCA) for dimensional reduction was performed based on the variable genes previously identified and cells are clustered using uniform manifold approximation and projection for dimensions reduction (UMAP) algorithm. Differentially expressed gene (DEG) analysis and cell pseudotime trajectory analysis were performed to identify biological pathway changes and differentiation states of EC subpopulations.
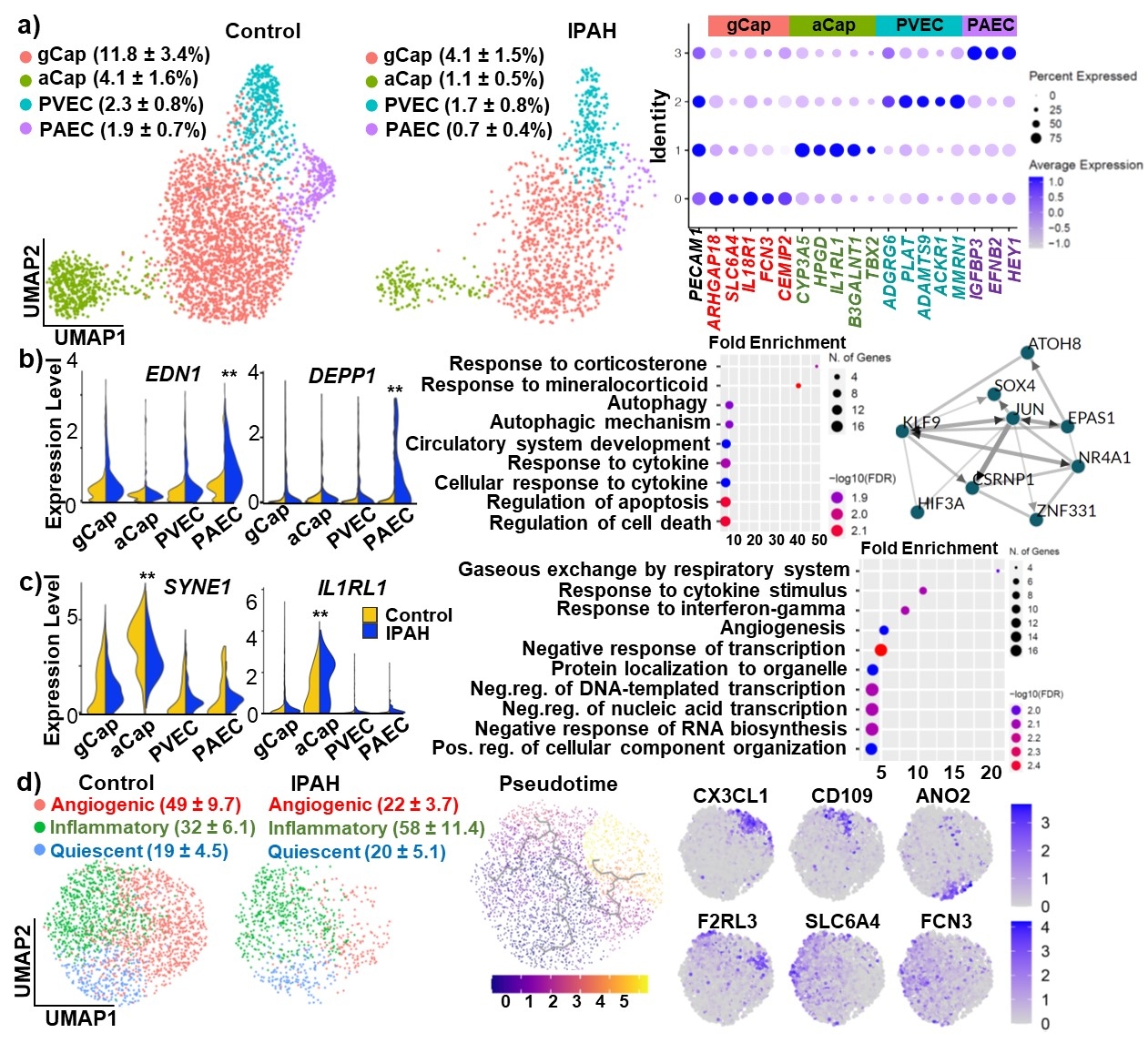
Results: Our data revealed 4 distinct EC subsets in human lungs: general capillary EC (gCap), alveolar specific capillary EC (aCap), pulmonary venous EC (PVEC), and pulmonary arterial EC (PAEC). The EC proportion in the whole lung diminished markedly in IPAH patients. Differentially expressed gene (DEG) analysis led to identification of IPAH-related biological process in different EC subsets including aberrant vasoconstriction and cell autophagy in PAEC. The function of aCap in IPAH lungs was skewed from a pro-gas/substance exchange to a pro-inflammation state. Transcriptionally distinct gCap subpopulations were enriched in specific biological processes and could be distinguished into 3 major clusters: angiogenic gCap, inflammatory gCap, and quiescent gCap. Moreover, we identified a skewed cellular distribution from the angiogenic gCap to inflammatory gCap in IPAH lungs.
Conclusions: The current study complements efforts to establish a lung cellular atlas and provides a blueprint of molecular/cellular changes associated with EC dysfunction in IPAH at single-nucleus level. EC dysfunction in the pathogenesis of IPAH is centered on EC phenotypic shift and skewed intercellular signaling pattern. Thus, targeting the genes that underlying the EC identity shift and dedifferentiation process may serve as a novel therapeutic strategy to reverse pulmonary vascular remodeling in PAH.
Methods: We utilized single-nucleus RNA sequencing (snRNAseq) to provide transcriptome insights into cellular composition changes within idiopathic PAH (IPAH) and donor lungs. Principal component analysis (PCA) for dimensional reduction was performed based on the variable genes previously identified and cells are clustered using uniform manifold approximation and projection for dimensions reduction (UMAP) algorithm. Differentially expressed gene (DEG) analysis and cell pseudotime trajectory analysis were performed to identify biological pathway changes and differentiation states of EC subpopulations.
Results: Our data revealed 4 distinct EC subsets in human lungs: general capillary EC (gCap), alveolar specific capillary EC (aCap), pulmonary venous EC (PVEC), and pulmonary arterial EC (PAEC). The EC proportion in the whole lung diminished markedly in IPAH patients. Differentially expressed gene (DEG) analysis led to identification of IPAH-related biological process in different EC subsets including aberrant vasoconstriction and cell autophagy in PAEC. The function of aCap in IPAH lungs was skewed from a pro-gas/substance exchange to a pro-inflammation state. Transcriptionally distinct gCap subpopulations were enriched in specific biological processes and could be distinguished into 3 major clusters: angiogenic gCap, inflammatory gCap, and quiescent gCap. Moreover, we identified a skewed cellular distribution from the angiogenic gCap to inflammatory gCap in IPAH lungs.
Conclusions: The current study complements efforts to establish a lung cellular atlas and provides a blueprint of molecular/cellular changes associated with EC dysfunction in IPAH at single-nucleus level. EC dysfunction in the pathogenesis of IPAH is centered on EC phenotypic shift and skewed intercellular signaling pattern. Thus, targeting the genes that underlying the EC identity shift and dedifferentiation process may serve as a novel therapeutic strategy to reverse pulmonary vascular remodeling in PAH.
More abstracts on this topic:
General Capillary Endothelial Cells Undergo Reprogramming into Arterial Endothelial Cells in Pulmonary Hypertension
Liu Bin, Yi Dan, Xia Xiaomei, Ramirez Karina, Zhao Hanqiu, Tripathi Ankit, Fallon Michael, Dai Zhiyu
Analysis of First Morning Urine Transcriptomes in Normotensive and Hypertensive Patients Identify Upregulated Inflammatory and Signaling Pathways Associated with HypertensionUmanath Kausik, Ortiz Pablo, Sohaney Ryann, Atchison Douglas, Abraham Emmy, Meng Ze, She Ruicong, Adrianto Indra, Levin Albert, Wu Andrew