Final ID: Mo3135
Limitations of the Generalizability of Semaglutide Trials Evaluating CV Outcomes
Abstract Body (Do not enter title and authors here): Introduction
In the SUSTAIN-6, SELECT, and FLOW clinical trials, semaglutide improved CV outcomes for primary and secondary prevention among those with poor cardiovascular-kidney-metabolic (CKM) health. However, whether trial participants are representative of the treatment-eligible U.S. population is uncertain.
Research Question:
How well do the SUSTAIN-6, SELECT, and FLOW trial populations each represent their respective treatment-eligible U.S. populations?
Methods:
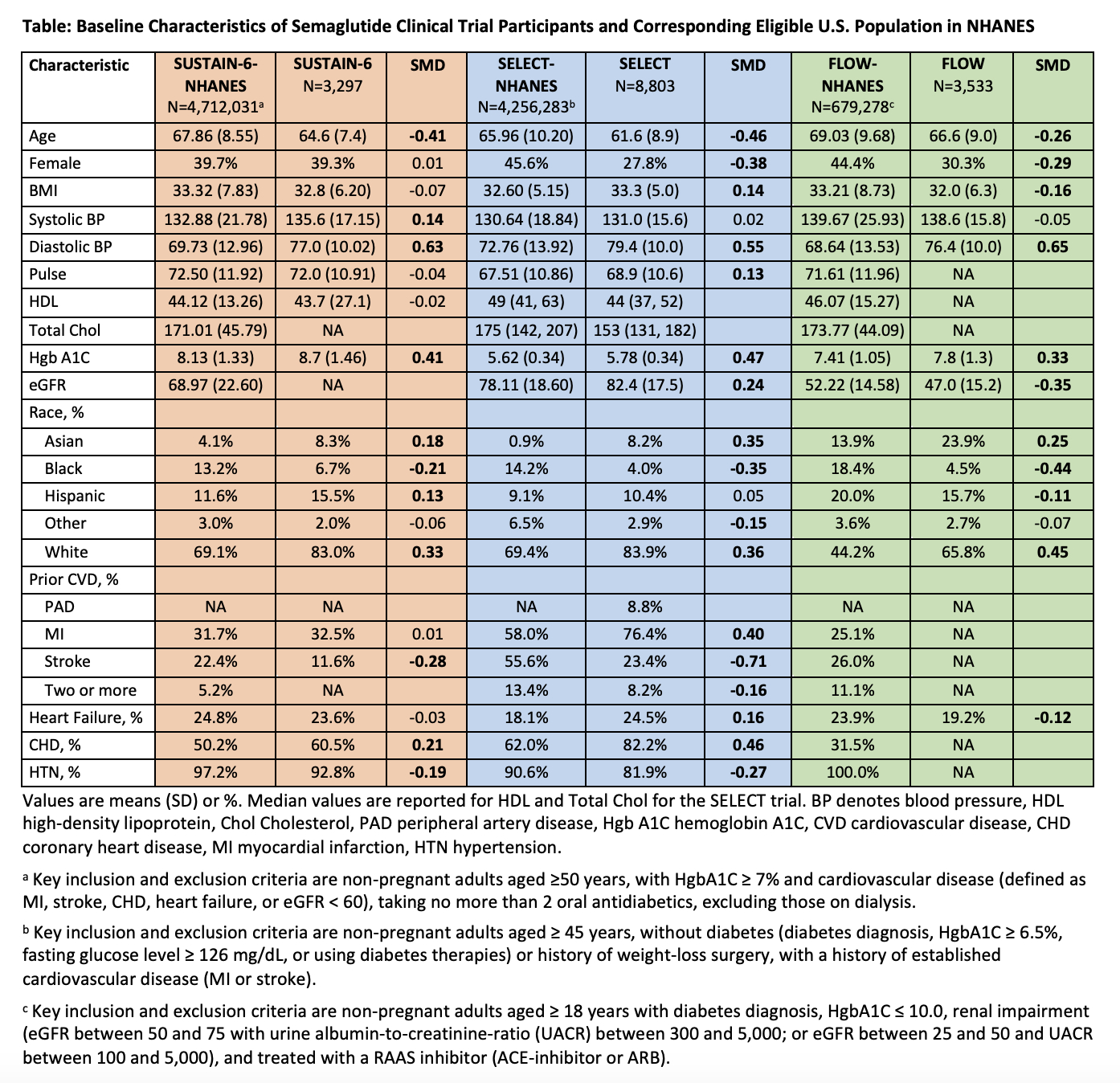
We identified U.S. adults eligible for semaglutide by applying key inclusion and exclusion criteria of the SUSTAIN-6, SELECT, and FLOW trials to the 2015 to March 2020 cycles of the National Health and Nutrition Examination Survey (NHANES). We compared baseline demographic and clinical characteristics of treatment-eligible NHANES populations (“real-world”) with those of their corresponding clinical trial populations using standardized mean differences (SMD). An absolute SMD ≥ 0.10 represents an imbalance in a given demographic or clinical characteristic between the trial and U.S. population.
Results:
Across the 3 trials, 39 of the 50 characteristics had an absolute SMD value ≥ 0.10 (Table). Trial participants were younger (SMD < -0.2) than the real-world populations. Women were significantly underrepresented in the SELECT and FLOW trials (SMD < -0.2). Self-reported race/ethnicity representation was imbalanced in all studies, with underrepresentation of Black (SMD < -0.2) individuals and Hispanic individuals in FLOW (SMD = -0.11). Trial subjects had higher diastolic blood pressure and HgbA1C. The greatest imbalance was that SELECT enrolled a lower proportion of patients with a history of stroke compared with the real-world population (a subgroup in which semaglutide showed equivocal CV benefit).
Conclusion
Of the three semaglutide CV outcome trials that have been published, all were not representative of the treatment-eligible U.S. population. The underrepresentation of women and Black and Hispanic individuals is notable because of the disproportionate burden of poor CKM health in these groups. Evaluation of semaglutide in representative, diverse US populations is needed to examine safety, effectiveness, and cost-effectiveness in the real-world.
In the SUSTAIN-6, SELECT, and FLOW clinical trials, semaglutide improved CV outcomes for primary and secondary prevention among those with poor cardiovascular-kidney-metabolic (CKM) health. However, whether trial participants are representative of the treatment-eligible U.S. population is uncertain.
Research Question:
How well do the SUSTAIN-6, SELECT, and FLOW trial populations each represent their respective treatment-eligible U.S. populations?
Methods:
We identified U.S. adults eligible for semaglutide by applying key inclusion and exclusion criteria of the SUSTAIN-6, SELECT, and FLOW trials to the 2015 to March 2020 cycles of the National Health and Nutrition Examination Survey (NHANES). We compared baseline demographic and clinical characteristics of treatment-eligible NHANES populations (“real-world”) with those of their corresponding clinical trial populations using standardized mean differences (SMD). An absolute SMD ≥ 0.10 represents an imbalance in a given demographic or clinical characteristic between the trial and U.S. population.
Results:
Across the 3 trials, 39 of the 50 characteristics had an absolute SMD value ≥ 0.10 (Table). Trial participants were younger (SMD < -0.2) than the real-world populations. Women were significantly underrepresented in the SELECT and FLOW trials (SMD < -0.2). Self-reported race/ethnicity representation was imbalanced in all studies, with underrepresentation of Black (SMD < -0.2) individuals and Hispanic individuals in FLOW (SMD = -0.11). Trial subjects had higher diastolic blood pressure and HgbA1C. The greatest imbalance was that SELECT enrolled a lower proportion of patients with a history of stroke compared with the real-world population (a subgroup in which semaglutide showed equivocal CV benefit).
Conclusion
Of the three semaglutide CV outcome trials that have been published, all were not representative of the treatment-eligible U.S. population. The underrepresentation of women and Black and Hispanic individuals is notable because of the disproportionate burden of poor CKM health in these groups. Evaluation of semaglutide in representative, diverse US populations is needed to examine safety, effectiveness, and cost-effectiveness in the real-world.
More abstracts on this topic:
A Randomized Clinical Trial for Asymptomatic Elevated Blood Pressure in Patients Discharged from Emergency Department
Prendergast Heather, Khosla Shaveta, Kitsiou Spyros, Petzel Gimbar Renee, Freels Sally, Sanders Anissa, Daviglus Martha, Carter Barry, Del Rios Marina, Heinert Sara
A randomized controlled trial of antithrombotic therapy in ischemic stroke patients with non-valvular atrial fibrillation and atherosclerosis: The ATIS-NVAF trialOkazaki Shuhei, Uchida Kazutaka, Asakura Koko, Omae Katsuhiro, Yamamoto Haruko, Hirano Teruyuki, Toyoda Kazunori, Iguchi Yasuyuki, Noguchi Teruo, Okada Yasushi, Kitagawa Kazuo, Tanaka Kanta, Sakai Nobuyuki, Yamagami Hiroshi, Yazawa Yukako, Doijiri Ryosuke, Koga Masatoshi, Ihara Masafumi, Yamamoto Shiro, Kamiyama Kenji, Honda Yuko