Final ID: Sa2172
Electronegative VLDL Impairs Mitochondrial Function and Promotes Cardiomyocyte Dysfunction in Heart Failure with Preserved Ejection Fraction
Abstract Body (Do not enter title and authors here): Background
Heart failure with preserved ejection fraction (HFpEF) affects energy metabolism, increasing reliance on long chain fatty acids (LCFA). Alterations to VLDL, the main triglyceride transporter, may significantly influence disease progression. VLDL can be separated into 5 subfractions from V1 (most beneficial, least electronegative) to V5 (most electronegative). This study investigates whether VLDL electronegativity induces cardiomyocyte dysfunction through changes in cellular metabolism.
Goals
We aim to explore the cellular mechanisms of V5-induced pathology in rat neonatal cardiomyocytes (RNC) and bovine aortic endothelial cells (BAEC), potentially accelerating the systemic inflammation that leads to the development of HFpEF.
Methods
V1 and V5 were isolated from healthy blood bank donors. RNCs were treated with PBS (control), V1, or V5 at 25 or 50 µg/mL for 24 hours. Mitochondrial function was evaluated using the Seahorse ATP production rate, cell mito stress, and palmitate oxidation stress test assays. Immunofluorescence staining of RNCs and BAECs was performed to elucidate systemic inflammation. Comprehensive lipidomics characterized components potentially involved in V5’s cytotoxic effects.
Results
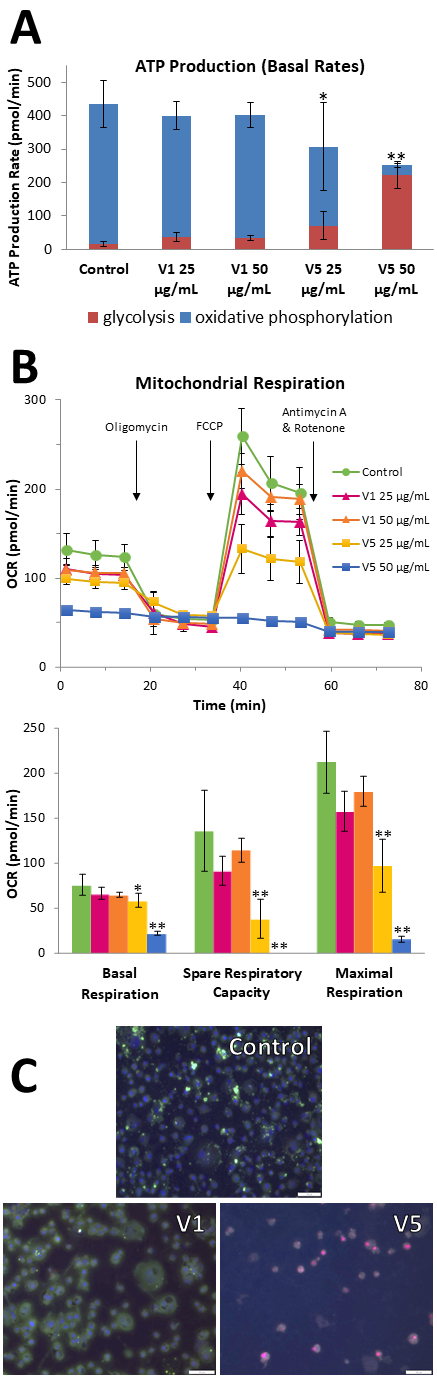
For RNCs treated with V5, ATP production shifted remarkably towards glycolysis instead of oxidative phosphorylation (Figure A). V5 but not V1 severely impaired mitochondrial respiration, affecting basal respiration, spare respiratory capacity, and maximal respiration in a concentration-dependent manner (Figure B). When LCFA transport into mitochondria is blocked, V5-treated RNCs showed increased cellular uptake of palmitate, leading to heightened lipotoxicity. In both RNCs and BAECs, V5 but not V1 reduced cell viability and increased apoptosis (Figure C). Lipidomic studies revealed that compared to V1, V5 contains multiple glycated lipid components and a richer triglyceride content.
Conclusion
We report novel findings that increased VLDL electronegativity provokes mitochondrial dysfunction through energy depletion and lipid overload, resulting in cardiomyocyte impairment. This explains how V5-rich VLDL, observed in metabolic syndrome, contributes to systemic inflammation and clinically manifests as HFpEF.
Heart failure with preserved ejection fraction (HFpEF) affects energy metabolism, increasing reliance on long chain fatty acids (LCFA). Alterations to VLDL, the main triglyceride transporter, may significantly influence disease progression. VLDL can be separated into 5 subfractions from V1 (most beneficial, least electronegative) to V5 (most electronegative). This study investigates whether VLDL electronegativity induces cardiomyocyte dysfunction through changes in cellular metabolism.
Goals
We aim to explore the cellular mechanisms of V5-induced pathology in rat neonatal cardiomyocytes (RNC) and bovine aortic endothelial cells (BAEC), potentially accelerating the systemic inflammation that leads to the development of HFpEF.
Methods
V1 and V5 were isolated from healthy blood bank donors. RNCs were treated with PBS (control), V1, or V5 at 25 or 50 µg/mL for 24 hours. Mitochondrial function was evaluated using the Seahorse ATP production rate, cell mito stress, and palmitate oxidation stress test assays. Immunofluorescence staining of RNCs and BAECs was performed to elucidate systemic inflammation. Comprehensive lipidomics characterized components potentially involved in V5’s cytotoxic effects.
Results
For RNCs treated with V5, ATP production shifted remarkably towards glycolysis instead of oxidative phosphorylation (Figure A). V5 but not V1 severely impaired mitochondrial respiration, affecting basal respiration, spare respiratory capacity, and maximal respiration in a concentration-dependent manner (Figure B). When LCFA transport into mitochondria is blocked, V5-treated RNCs showed increased cellular uptake of palmitate, leading to heightened lipotoxicity. In both RNCs and BAECs, V5 but not V1 reduced cell viability and increased apoptosis (Figure C). Lipidomic studies revealed that compared to V1, V5 contains multiple glycated lipid components and a richer triglyceride content.
Conclusion
We report novel findings that increased VLDL electronegativity provokes mitochondrial dysfunction through energy depletion and lipid overload, resulting in cardiomyocyte impairment. This explains how V5-rich VLDL, observed in metabolic syndrome, contributes to systemic inflammation and clinically manifests as HFpEF.
More abstracts on this topic:
A Highly Selective and Orally Available HDAC6 Inhibitor, EKZ-102, Ameliorates Cardiac Dysfunction and Exercise Intolerance in Cardiometabolic HFpEF
Elbatreek Mahmoud, Goodchild Traci, Lefer David, Evans Lauren, Richardson Thomas, James Rebecca, Schroeder Frederick, Wang Jianhong, Luterman Jim, Gilbert Tonya, Fisher Richard
Association Between Myocardial Lipomatous Metaplasia And Sudden Cardiac Death In Patients With Prior Myocardial InfarctionAisikaier Kaisaierjiang