Final ID: MDP1269
Evaluation of 3D Bioprinted Pulsatile Conduits Engineered Using Human Stem Cell-Derived Cardiomyocytes in a Murine Model
Abstract Body (Do not enter title and authors here): Introduction
Patients with single ventricle disease and Fontan circulation suffer long-term morbidity due to the lack of a sub-pulmonary ventricle. The concept of a pulsatile Fontan conduit, functioning as a sub-pulmonary ‘neo-ventricle,’ using engineered heart tissue (EHT), may be a potential solution. To this end, we developed a tissue-engineered vascular conduit with EHT that allowed surgical implantation into a murine model for in-vivo assessment.
Methods
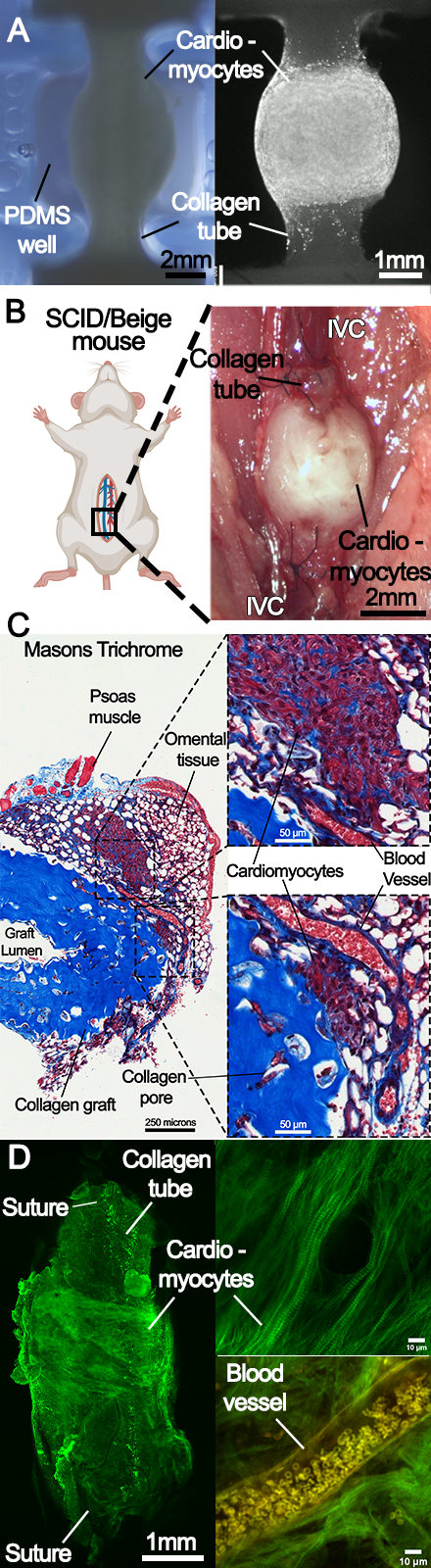
Mouse-scale conduits were fabricated from type I collagen using freeform reversible embedding of suspended hydrogels (FRESH) 3D bioprinting. The conduits were cellularized using human stem cell-derived cardiomyocytes and cardiac fibroblasts. Electrophysiology was assessed using calcium imaging. The conduits were surgically anastomosed to the infra-renal inferior vena cava (IVC) as an end-end interposition graft in severe combined immunodeficiency (SCID)/beige mice. The conduit's patency and the EHT's contractility were monitored with monthly ultrasounds. Explanted conduits underwent histology, immunohistochemistry (IHC), and immunofluorescent (IF) imaging.
Results
Sixteen contractile conduits were produced. In vitro calcium imaging demonstrated anisotropic calcium wave propagation along the conduit at day 14. Twelve grafts were implanted in the mouse IVC and half of the grafts (n=6) were explanted 6 months postoperatively. At explant, five showed synchronous contractile behavior in-vivo of the EHT around the collagen conduit. Interconnected networks of sarcomeric alpha actinin-positive cardiomyocytes were observed on IHC and IF. Neo-vascularization was seen infiltrating the EHT on histology. Six mice remained alive at 11 months post-operatively, three with contractile activity seen via ultrasound. All twelve grafts were patent on ultrasound with no stenosis.
Conclusion
We have produced mouse-scale pulsatile conduits using human embryonic-derived cardiomyocytes and bioprinting that are robust enough to withstand surgical anastomosis to the IVC and remain patent. We observed functional beating of the EHT at 11 months in vivo. This model could be used to assess and improve the in-vivo function of EHT towards producing a larger-scale pulsatile Fontan conduit.
Patients with single ventricle disease and Fontan circulation suffer long-term morbidity due to the lack of a sub-pulmonary ventricle. The concept of a pulsatile Fontan conduit, functioning as a sub-pulmonary ‘neo-ventricle,’ using engineered heart tissue (EHT), may be a potential solution. To this end, we developed a tissue-engineered vascular conduit with EHT that allowed surgical implantation into a murine model for in-vivo assessment.
Methods
Mouse-scale conduits were fabricated from type I collagen using freeform reversible embedding of suspended hydrogels (FRESH) 3D bioprinting. The conduits were cellularized using human stem cell-derived cardiomyocytes and cardiac fibroblasts. Electrophysiology was assessed using calcium imaging. The conduits were surgically anastomosed to the infra-renal inferior vena cava (IVC) as an end-end interposition graft in severe combined immunodeficiency (SCID)/beige mice. The conduit's patency and the EHT's contractility were monitored with monthly ultrasounds. Explanted conduits underwent histology, immunohistochemistry (IHC), and immunofluorescent (IF) imaging.
Results
Sixteen contractile conduits were produced. In vitro calcium imaging demonstrated anisotropic calcium wave propagation along the conduit at day 14. Twelve grafts were implanted in the mouse IVC and half of the grafts (n=6) were explanted 6 months postoperatively. At explant, five showed synchronous contractile behavior in-vivo of the EHT around the collagen conduit. Interconnected networks of sarcomeric alpha actinin-positive cardiomyocytes were observed on IHC and IF. Neo-vascularization was seen infiltrating the EHT on histology. Six mice remained alive at 11 months post-operatively, three with contractile activity seen via ultrasound. All twelve grafts were patent on ultrasound with no stenosis.
Conclusion
We have produced mouse-scale pulsatile conduits using human embryonic-derived cardiomyocytes and bioprinting that are robust enough to withstand surgical anastomosis to the IVC and remain patent. We observed functional beating of the EHT at 11 months in vivo. This model could be used to assess and improve the in-vivo function of EHT towards producing a larger-scale pulsatile Fontan conduit.
More abstracts on this topic:
Bismuth Nanoparticle-Infused Bioresorbable Graft Enables Multimodal Computed Tomography and Photoacoustic Imaging-Based Monitoring and Promotes Vascular Regeneration
Barcena Allan John, Fowlkes Natalie, Bouchard Richard, Huang Steven, Melancon Marites, Bernardino Marvin, Mishra Archana, Bolinas Dominic Karl, Marco Kitz Paul, Fernandez Kim Claudette, San Valentin Erin Marie, Court Karem, Godin Biana
Fontan-Associated Liver Disease On A ChipRezapourdamanab Sarah, Dasi Lakshmi, Romero Rene, Bauser Heaton Holly, Serpooshan Vahid, Singh Yamini, Norton Sophia, Hwang Boeun, Jin Linqi, Salar Amoli Mehdi, Karnik Shweta, Avazmohammadi Reza, Neelakantan Sunder