Final ID: Su2159
Mode of Death in Heart Failure with Preserved Ejection across the Kidney Function Spectrum: Pooled Individual-Patient Level Analysis of 5 Trials
Methods: We leveraged individual patient level data from 5 trials of HF with mildly reduced or preserved ejection (CHARM-Preserved, I Preserve, TOPCAT [Americas region], PARAGON-HF, and DELIVER). Causes of death (sudden, heart failure, other CV, and non-CV) were adjudicated by clinical events committees in each respective trial.
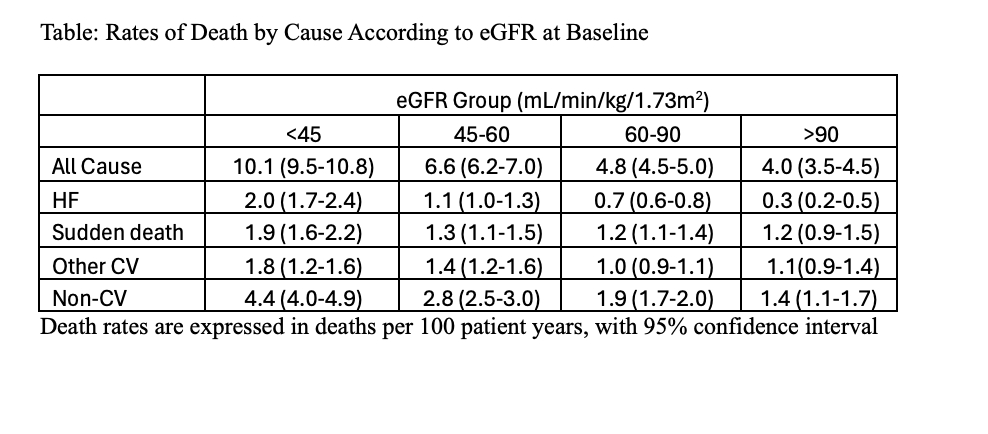
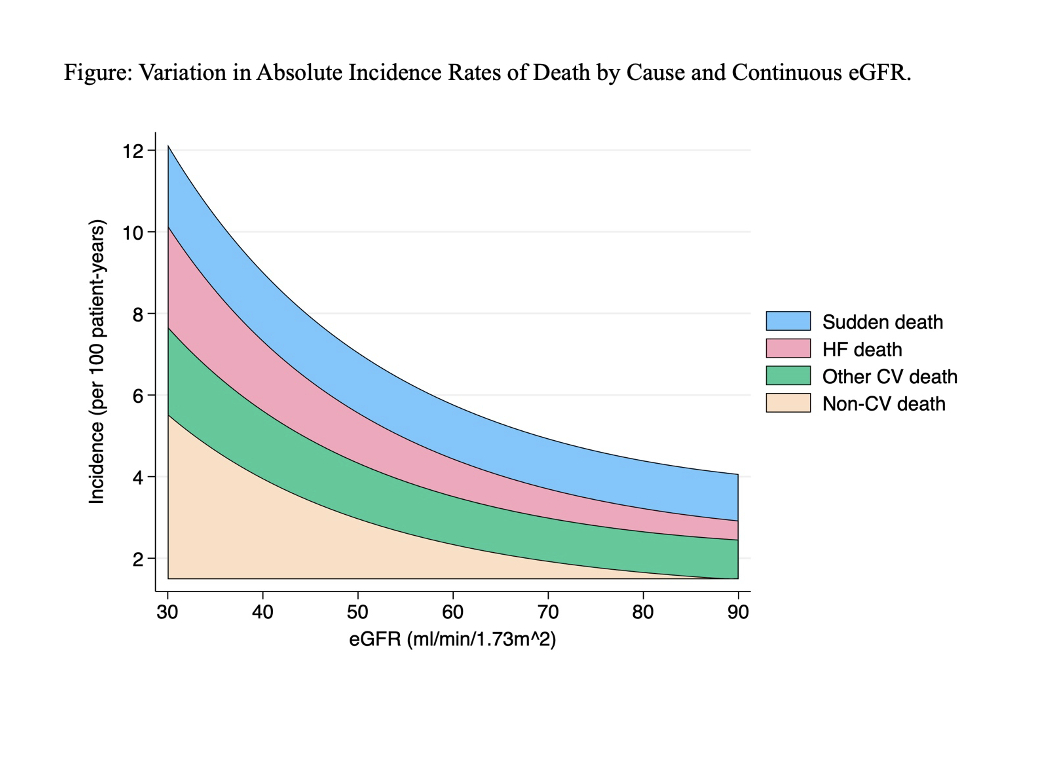
Results: Among 17,947 patients across the 5 trials with available eGFR data, mean age was 71.6 ± 9.0 years, 51% were women, median NT-proBNP was 840 [25-75th percentile 424, 1566] pg/ml. Overall, 2084 (12%) had eGFR ≥90 mL/min/1.73m2, 7977 (44%) had eGFR 60 - < 90, 4701 (26%) had eGFR 45-60, 3185 (18%) had eGFR <45. During a mean of 2.9 years of follow-up, 3,140 patients died. All-cause death rate was greater in the lower eGFR groups, driven by greater rates of HF and non-CV death. Rates of CV and non-CV death were 5.7/100 patient years (py) and 4.4/100py in patients with eGFR <45 and 2.7/100py and 1.3/100py in patients with eGFR >90 (Table and Figure). HF-related death rate was markedly greater in participants with eGFR <45 (2.0/100py) compared to patients with eGFR>90 (0.3/100py)
Conclusions: Among nearly 18,000 patients across contemporary HFmrEF/HFpEF clinical trials, mortality was markedly higher at lower ranges of kidney function, driven mostly by higher non-CV death and HF-related death.
- Bart, Nicole ( Brigham and Women's Hospital , Boston, MA , Massachusetts , United States )
- Pfeffer, Marc ( BRIGHAM and WOMENS HOSPITAL , Boston , Massachusetts , United States )
- Pitt, Bertram ( UNIVERITY HOSPITAL , Ann Arbor , Michigan , United States )
- Zannad, Faiez ( CVCT and Universite de Lorraine , Paris , France )
- Zile, Michael ( MEDICAL UNIV OF SOUTH CAROLINA , Charleston , South Carolina , United States )
- Mcmurray, John ( BHF CARDIOVASCULAR RESEARCH CENTRE , Glasgow , United Kingdom )
- Solomon, Scott ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Cunningham, Jonathan ( Brigham and Womens Hospital , Chestnut Hill , Massachusetts , United States )
- Ariss, Robert ( Brigham and Women's Hospital , Boston, MA , Massachusetts , United States )
- Claggett, Brian ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Vaduganathan, Muthiah ( Brigham and Womens Hospital , Boston , Massachusetts , United States )
- Neuen, Brendon ( George Institute for Global Health , Newtown , New South Wales , Australia )
- Beldhuis, Iris ( UMCG , Groningen , Netherlands )
- Desai, Akshay ( BRIGHAM WOMENS HOSPITAL , Boston , Massachusetts , United States )
- Jhund, Pardeep ( UNIVERSITY OF GLASGOW , Glasgow , United Kingdom )
- Mc Causland, Finnian ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
Meeting Info:
Session Info:
Cracking Comorbidities and Complications in Heart Failure
Sunday, 11/17/2024 , 11:30AM - 12:30PM
Abstract Poster Session
More abstracts on this topic:
Pergola Pablo, Szarek Michael, Zayed Hany, Hemani Famina, Degoma Emil, Andorfer Cathy, Walsh John, Ridker Paul, Bhatt Deepak
A novel Urocortin-2 analog COR-1167 corrects cardiac and renal dysfunction on top of Empagliflozin in a rat model of acute decompensated heart failureStephan Yohan, Corruble Clement, Charrier Lucie, Nicol Lionel, Kowala Mark, Ozoux Marie-laure, Lawson Francesca, Janiak Philip, Mulder Paul
More abstracts from these authors:
Lu Henri, Pfeffer Marc, Pitt Bertram, Zannad Faiez, Zile Michael, Mcmurray John, Solomon Scott, Desai Akshay, Kondo Toru, Claggett Brian, Vaduganathan Muthiah, Neuen Brendon, Beldhuis Iris, Jhund Pardeep, Mc Causland Finnian, Anand Inder
Systolic Blood Pressure in Heart Failure with Mildly Reduced or Preserved Ejection Fraction: A Pooled Participant-Level Analysis of 4 Large-Scale TrialsLu Henri, Pfeffer Marc, Pitt Bertram, Zannad Faiez, Zile Michael, Mcmurray John, Solomon Scott, Desai Akshay, Kondo Toru, Claggett Brian, Vaduganathan Muthiah, Neuen Brendon, Beldhuis Iris, Jhund Pardeep, Mc Causland Finnian, Anand Inder