Final ID: MDP139
Efficacy and Safety of Rivaroxaban Versus Low Molecular Weight Heparin for Treating Venous Thromboembolism in Cancer Patients: A Systematic Review and Meta-Analysis
Abstract Body (Do not enter title and authors here): Background: Rivaroxaban is a novel oral anticoagulant suggested as an alternative to low molecular weight heparin (LMWH). However, its efficacy and safety compared to LMWH for treating venous thromboembolism (VTE) in cancer patients remain unclear.
Hypothesis: This study aims to compare the efficacy and safety of rivaroxaban against LMWH for treating VTE in cancer patients.
Methods: We conducted a literature search for relevant articles on PubMed, Google Scholar, and Embase. Outcomes were pooled using the DerSimonian and Laird random-effects model as risk ratios (RR) with 95% confidence intervals (CI). A p-value of <0.05 was considered statistically significant.
Results: A total of 14368 cancer patients (Rivaroxaban: 4378 and LMWH: 9990) from 13 studies were included with an average age of 62.5 and 61.9 respectively.
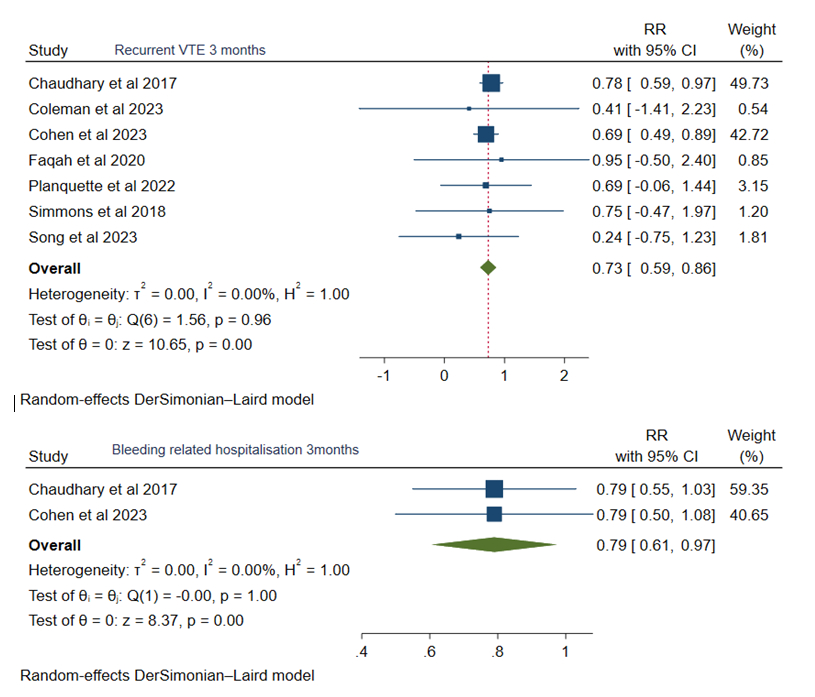
At 3 months, rivaroxaban was associated with a decreased risk of recurrent VTE (RR 0.73, 95% CI: 0.59, 0.86, p<0.05) and bleeding-related hospitalization (RR 0.79, 95% CI: 0.61, 0.97, p<0.05) with statistical signifcant whereas, major bleeding (RR 0.87, 95% CI: 0.09, 1.65), non-major bleeding (RR 0.84, 95% CI: 0.60, 1.08) and all-cause mortality (RR 0.91, 95% CI: 0.72, 1.09) were comparable.
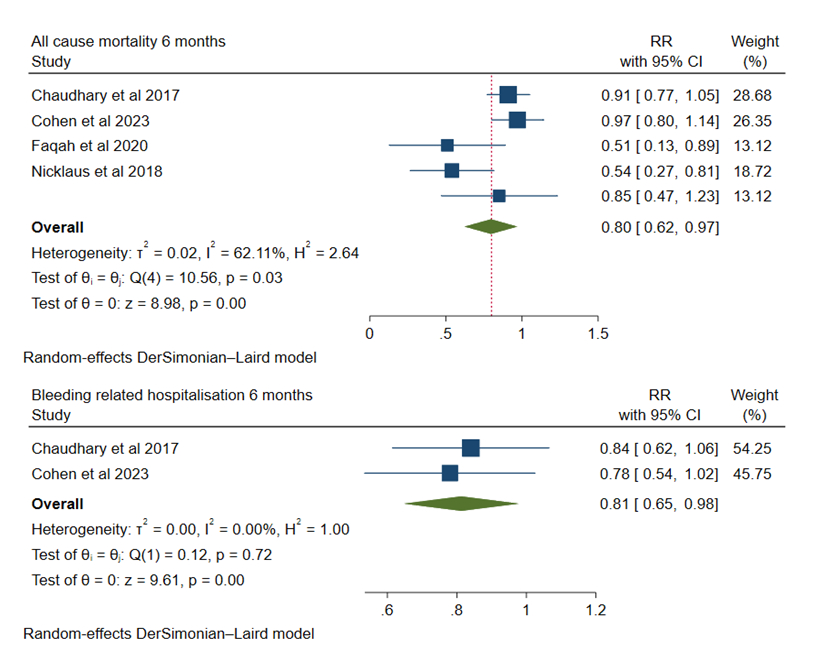
At 6 months, rivaroxaban was associated with a decreased risk of all-cause mortality (RR 0.80, 95% CI: 0.62, 0.97, p<0.05), and bleeding-related hospitalization (RR 0.81, 95% CI: 0.65, 0.98, p<0.05) whereas major bleeding (RR 0.88, 95% CI: 0.52, 1.24), non-major bleeding (RR 0.85, 95% CI: 0.42, 1.29) and recurrent VTE (RR 0.83, 95% CI: 0.64, 1.01) were comparable.
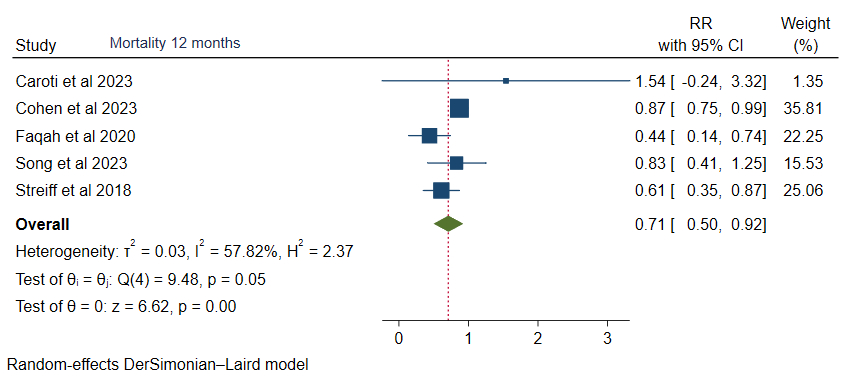
At 12 months, rivaroxaban was associated with a decreased risk of mortality (RR 0.71, 95% CI: 0.50, 0.92, p<0.05), however non-major bleeding (RR 1.38, 95% CI: 0.83, 1.94), cancer-associated thrombosis recurrence (RR 0.94, 95% CI: 0.73, 1.16), and bleeding (RR 0.70, 95% CI: 0.26, 1.15) were comparable.
Conclusion: While rivaroxaban is associated with low adverse outcomes suggesting its use in treating VTE in cancer patients, further research is recommended.
Hypothesis: This study aims to compare the efficacy and safety of rivaroxaban against LMWH for treating VTE in cancer patients.
Methods: We conducted a literature search for relevant articles on PubMed, Google Scholar, and Embase. Outcomes were pooled using the DerSimonian and Laird random-effects model as risk ratios (RR) with 95% confidence intervals (CI). A p-value of <0.05 was considered statistically significant.
Results: A total of 14368 cancer patients (Rivaroxaban: 4378 and LMWH: 9990) from 13 studies were included with an average age of 62.5 and 61.9 respectively.
At 3 months, rivaroxaban was associated with a decreased risk of recurrent VTE (RR 0.73, 95% CI: 0.59, 0.86, p<0.05) and bleeding-related hospitalization (RR 0.79, 95% CI: 0.61, 0.97, p<0.05) with statistical signifcant whereas, major bleeding (RR 0.87, 95% CI: 0.09, 1.65), non-major bleeding (RR 0.84, 95% CI: 0.60, 1.08) and all-cause mortality (RR 0.91, 95% CI: 0.72, 1.09) were comparable.
At 6 months, rivaroxaban was associated with a decreased risk of all-cause mortality (RR 0.80, 95% CI: 0.62, 0.97, p<0.05), and bleeding-related hospitalization (RR 0.81, 95% CI: 0.65, 0.98, p<0.05) whereas major bleeding (RR 0.88, 95% CI: 0.52, 1.24), non-major bleeding (RR 0.85, 95% CI: 0.42, 1.29) and recurrent VTE (RR 0.83, 95% CI: 0.64, 1.01) were comparable.
At 12 months, rivaroxaban was associated with a decreased risk of mortality (RR 0.71, 95% CI: 0.50, 0.92, p<0.05), however non-major bleeding (RR 1.38, 95% CI: 0.83, 1.94), cancer-associated thrombosis recurrence (RR 0.94, 95% CI: 0.73, 1.16), and bleeding (RR 0.70, 95% CI: 0.26, 1.15) were comparable.
Conclusion: While rivaroxaban is associated with low adverse outcomes suggesting its use in treating VTE in cancer patients, further research is recommended.
More abstracts on this topic:
Acute Left Ventricular Thrombosis and Systemic Embolism Following Testosterone Therapy
Rasheed Ahmed Daniyaal, Naseer Ahmed, Nadig Vidya
Acoustic Analysis For The Detection Of Leaflet Thrombosis After Transcatheter Aortic Valve ReplacementLupu Lior, Shetrit Aviel, Kadosh Adi, Aviram Galit, Topilsky Yan, Banai Shmuel, Havakuk Ofer