Final ID: Su3024
Comparison of The Burden of Cardiovascular-Kidney-Metabolic Syndrome Components in Heart Failure with Preserved Ejection Fraction clinical trials and Heart Failure in the General United States Population
Aim: We sought to describe the prevalence of CKM components in HFpEF leveraging data from control participants in clinical trials and NHANES.
Methods: We analyzed data presented in publications from four randomized clinical trials that enrolled participants with HFpEF (all of which are available via NHLBI BioLINCC and will be included in the extant HeartShare dataset): TOPCAT, RELAX, NEAT-HFpEF, and INDIE. We abstracted baseline demographic and clinical variables from study publications to define CKM components (e.g., body mass index [BMI], diabetes status, hypertension, CKD status). We compared prevalence of CKM in these trials with a representative sample of US adults with self-reported history of heart failure (HF) from NHANES 2011-2018.
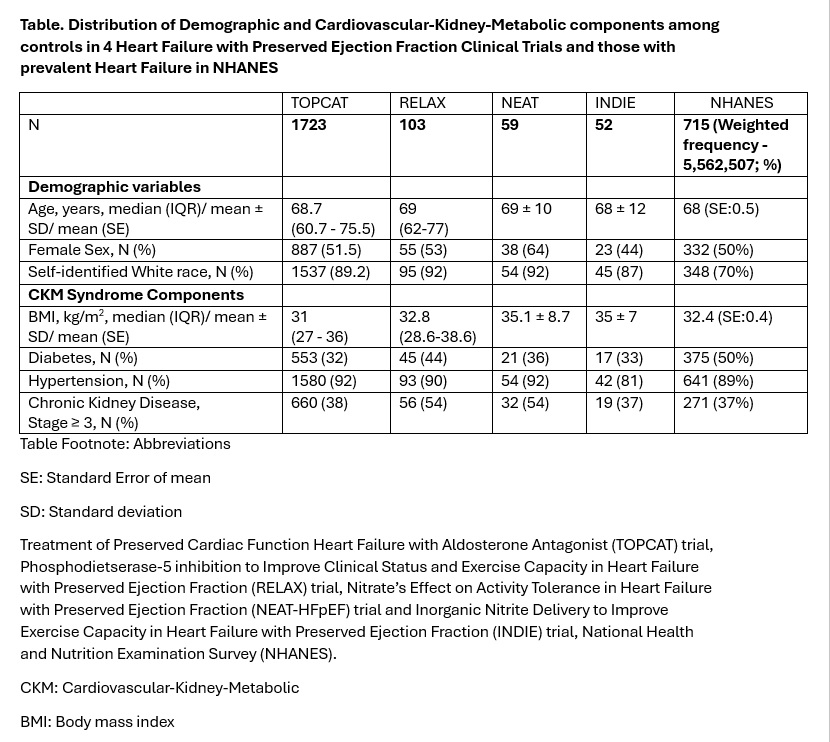
Results: We included 1937 patients with HFpEF from four trials with enrollment between 2006-2016 and 715 adults representing 5.6 million adults aged ≥40 years with a self-report of HF between 2011-2018 from NHANES (Table). Mean age was similar among enrolled trial participants with HFpEF and those with HF in NHANES (~68-69 years). Mean BMI was in the obesity range and was similar among trial participants with HFpEF and HF in NHANES (31-35 kg/m2). A significant proportion of individuals had hypertension in HFpEF trials (>80%) and in the NHANES sample with HF (89%). The proportion of stage ≥ 3 CKD in HFpEF trials (≥ 37%) was similar to the NHANES sample with HF (37%). Results are shown in Table.
Conclusions: Patients with HFpEF enrolled in clinical trials and those with HF in general population are significantly enriched with CKM components that may contribute to morbidity and mortality. Similar prevalence estimates were observed despite variability in trial inclusion criteria and heterogeneity in defining HFpEF and highlight CKM as a central target for HFpEF management.
- Hammond, Michael ( Northwestern University , Evanston , Illinois , United States )
- Lewis, Gregory ( Massachusetts General Hospital , Boston , Massachusetts , United States )
- Sharma, Kavita ( Johns Hopkins University SOM , Baltimore , Maryland , United States )
- Lopez, Javier ( UC DAVIS , Davis , California , United States )
- Shah, Sanjiv ( NORTHWESTERN UNIVERSITY , Chicago , Illinois , United States )
- Khan, Sadiya ( Northwestern University , Oak Park , Illinois , United States )
- Rasmussen-torvik, Laura ( NORTHWESTERN UNIVERSITY , Chicago , Illinois , United States )
- Cyrille-superville, Nicole ( Wake Forest University , Charlotte , North Carolina , United States )
- Givertz, Michael ( Brigham and Womens Hospital , Boston , Massachusetts , United States )
- Desai, Akshay ( BRIGHAM WOMENS HOSPITAL , Boston , Massachusetts , United States )
- Redfield, Margaret ( Mayo Clinic-Dr. Margaret Redfield , Rochester , Minnesota , United States )
- Chirinos, Julio ( University of Pennsylvania , Philadelphia , Pennsylvania , United States )
- Kitzman, Dalane ( WAKE FOREST BAPTIST HEALTH , Winston Salem , North Carolina , United States )
- Bertoni, Alain ( WAKE FOREST UNIV SCHOOL MED , Pfafftown , North Carolina , United States )
- Borlaug, Barry ( MAYO CLINIC , Rochester , Minnesota , United States )
Meeting Info:
Session Info:
Translating the Concept of CKM Syndrome to Real-World Populations
Sunday, 11/17/2024 , 03:15PM - 04:15PM
Abstract Poster Session
More abstracts on this topic:
Medina Jesse, Vincent Louis, Rodriguez Ferreira Esteban, Spence-miller Shanice, Fernandez Joel, Colombo Rosario, Calfa Marian
A Highly Selective and Orally Available HDAC6 Inhibitor, EKZ-102, Ameliorates Cardiac Dysfunction and Exercise Intolerance in Cardiometabolic HFpEFElbatreek Mahmoud, Goodchild Traci, Lefer David, Evans Lauren, Richardson Thomas, James Rebecca, Schroeder Frederick, Wang Jianhong, Luterman Jim, Gilbert Tonya, Fisher Richard
More abstracts from these authors:
Hammond Michael, Petito Lucia, Kazi Dhruv, Khan Sadiya, Huang Xiaoning
Improved Identification of Near-Term Heart Failure Risk in Pooled Cohort Studies Using ECG-AI and PREVENT-HFDesai Akshay, Pandey Ambarish, Suratekar Rohit, Alger Heather, Awasthi Samir, Ahmad Faraz, Oh Jae, Khan Sadiya, Shah Sanjiv