Final ID: Mo1008
Systemic Vascular Damage and Cardiac Dysfunction in a Mouse Severe Acute Pancreatitis Model
Abstract Body (Do not enter title and authors here): Background and Objective: Decline of cardiovascular (CV) function is commonly observed in patients with severe acute pancreatitis (AP), which is associated with high mortality and morbidity. While the pathological changes in the pancreas during severe AP have been extensively studied, the impact on the CV system has not been well characterized. Furthermore, obesity is a major risk factor for severe AP. Hereby we utilized an obesity-associated mouse AP model to assess potential alterations in vascular integrity and cardiac function, two major components of circulatory failure.
Methods: Wild-type male C57BL6J mice were fed a normal or a high fat (to induce obesity) diet. Both groups received intraperitoneal injection of cerulein (100 mg/kg/h x12) to induce AP. H&E and TUNEL staining of pancreas were performed to assess histology and cell death. To measure vascular integrity, we measured the leakiness of a fluorescein-conjugated dextran (70 kDa) in tissue parenchyma and serum levels of soluble vascular endothelial (VE)-cadherin, which has recently shown to be shed from endothelial cells with disrupted adherens junctions. Cardiac systolic and diastolic function were assessed by echocardiography at baseline and post-AP induction.
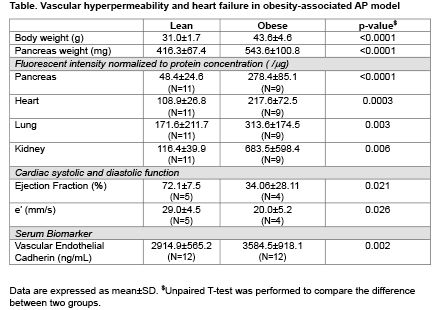
Results: Compared to lean mice, obese mice developed more severe pancreatic edema, cell death and inflammatory cell infiltration, along with significantly increased accumulation of fluorescent dextran in multiple organs including pancreas, heart, lung, and kidney, suggesting systemic disruption of vascular integrity. Increased serum levels of soluble VE-cadherin in obese mice (vs. lean) after AP induction further supported compromised endothelial cell barrier function. At baseline, obese mice exhibited similar cardiac function to lean mice. However, obese mice, but not lean mice, developed impaired systolic and diastolic function post-AP induction, as supported by decreased ejection fraction and e’ velocity respectively.
Conclusion: In this obesity-associated severe AP mouse model, systemic vascular permeability is increased, and cardiac function is substantially compromised. Systemic vascular hyperpermeability and cardiac dysfunction may represent therapeutic targets to enhance the outcomes in severe AP.
Methods: Wild-type male C57BL6J mice were fed a normal or a high fat (to induce obesity) diet. Both groups received intraperitoneal injection of cerulein (100 mg/kg/h x12) to induce AP. H&E and TUNEL staining of pancreas were performed to assess histology and cell death. To measure vascular integrity, we measured the leakiness of a fluorescein-conjugated dextran (70 kDa) in tissue parenchyma and serum levels of soluble vascular endothelial (VE)-cadherin, which has recently shown to be shed from endothelial cells with disrupted adherens junctions. Cardiac systolic and diastolic function were assessed by echocardiography at baseline and post-AP induction.
Results: Compared to lean mice, obese mice developed more severe pancreatic edema, cell death and inflammatory cell infiltration, along with significantly increased accumulation of fluorescent dextran in multiple organs including pancreas, heart, lung, and kidney, suggesting systemic disruption of vascular integrity. Increased serum levels of soluble VE-cadherin in obese mice (vs. lean) after AP induction further supported compromised endothelial cell barrier function. At baseline, obese mice exhibited similar cardiac function to lean mice. However, obese mice, but not lean mice, developed impaired systolic and diastolic function post-AP induction, as supported by decreased ejection fraction and e’ velocity respectively.
Conclusion: In this obesity-associated severe AP mouse model, systemic vascular permeability is increased, and cardiac function is substantially compromised. Systemic vascular hyperpermeability and cardiac dysfunction may represent therapeutic targets to enhance the outcomes in severe AP.
More abstracts on this topic:
ACLY Inhibition as a Novel Therapeutic Approach for Vascular Remodeling in Coronary Artery Disease.
Grobs Yann, Reem El-kabbout, Potus Francois, Provencher Steeve, Boucherat Olivier, Bonnet Sebastien, Romanet Charlotte, Lemay Sarah-eve, Bourgeois Alice, Voisine Pierre, Theberge Charlie, Sauvaget Melanie, Breuils Bonnet Sandra, Martineau Sandra
A Multicentre Study for Hands Only CPR (HOCPR) training assessment towards building a ‘Nation of Life Savers” in IndiaRavikumar Thanjavur, Sarma Kvs, Ravikumar Thanjavur, Sarkar Manuj, Debnath Dhrubajyoti, Behera Priyamadhaba, Ghate Jayshri, Trikha Divay, Samantaray A, Madhavi K