Final ID: Mo2194
Changes in EQ-5D-5L with Aficamten in Obstructive Hypertrophic Cardiomyopathy (oHCM): the SEQUOIA-HCM Trial
Hypothesis: Aficamten, compared with placebo, improves QoL as assessed by the EQ-5D-5L.
Aims: To describe EQ-5D-5L changes in patients enrolled in SEQUOIA-HCM.
Methods: SEQUOIA-HCM (NCT05186818) is a phase III, placebo-controlled trial of adults with symptomatic oHCM randomized to aficamten (n=142) or placebo (n=140) on top of standard-of-care medical therapy. Participants underwent blinded dose escalation over 6 weeks and were treated for 24 weeks followed by a 4-week washout. EQ-5D-5L (ranges from 0 to 1) and overall QoL using the Visual Analogue Scale (VAS; from 0 to 100) was measured at baseline through week 28 with higher scores indicating better QoL. Aficamten treatment effect on EQ-5D-5L was estimated using linear regression (study visits) and mixed effects regression (overall treatment effect) using all follow-up data with model adjustments for baseline EQ-5D-5L score and randomization stratification variables.
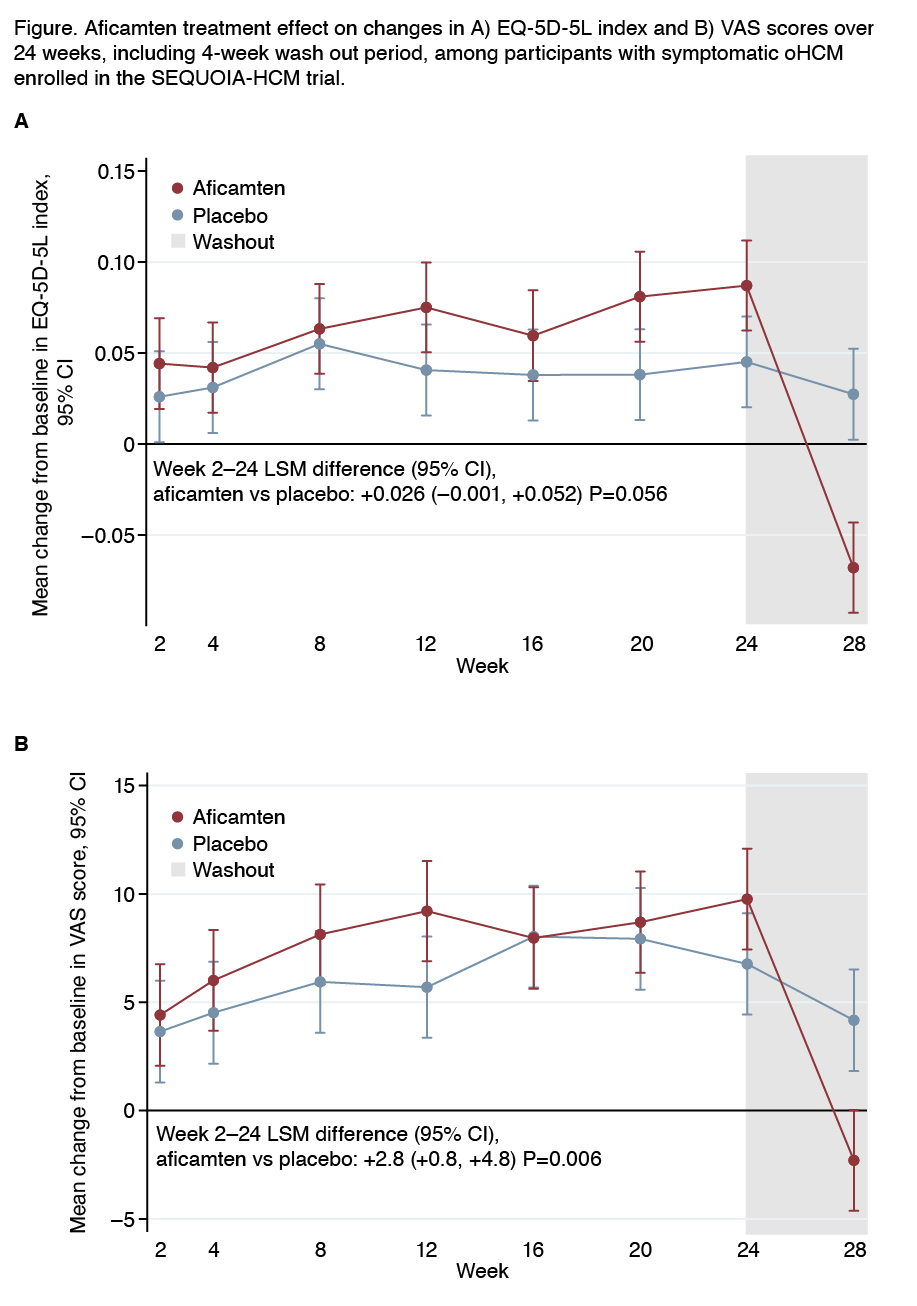
Results: Mean age (n=282) was 59.1±12.9 years (40.8% women; 79.1% white). Aficamten baseline EQ-5D-5L index and VAS scores were 0.8±0.19 and 71.2±17.9 (placebo: 0.78±0.2; 68.9±18.6), respectively. Aficamten improved the EQ-5D-5L by 0.026 (95% CI: -0.001, 0.052; p=0.056) and the VAS by 2.8 points (95% CI: 0.8, 4.8; p=0.006) versus placebo with significant differences as early as 8 weeks after treatment initiation (p=0.005). After treatment withdrawal at 24 weeks, QoL benefits in aficamten-treated patients decreased compared with placebo (Figure: Index, p<0.001; VAS, p=0.003), with hemodynamic parameters returning to baseline.
Conclusions: Aficamten yielded early, sustained and significant improvement in overall QoL compared with placebo. Withdrawal of aficamten was associated with the loss of treatment benefits and perhaps an increased perception of pre-treatment symptoms. These data support a role of aficamten in improving QoL among patients with oHCM.
- Schulze, Christian ( UNIVERSITY HOSPITAL JENA , Jena , Germany )
- Kwong, Raymond ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Maron, Martin ( Lahey Hospital and Medical Center , Burlington , Massachusetts , United States )
- Masri, Ahmad ( Oregon Health & Science University , Portland , Oregon , United States )
- Nassif, Michael ( University of Missouri Kansas City Healthcare Institute for Innovations in Quality and Saint Luke’s Mid America Heart Institute , Kansas City , Missouri , United States )
- Olivotto, Iacopo ( Meyer Children’s Hospital, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) , Florence , Italy )
- Spertus, John ( University of Missouri Kansas City Healthcare Institute for Innovations in Quality and Saint Luke’s Mid America Heart Institute , Kansas City , Missouri , United States )
- Veselka, Josef ( Motol University Hospital , Prague , Czechia )
- Butzner, Michael ( Cytokinetics , South San Francisco , California , United States )
- Heitner, Stephen ( Cytokinetics Inc. , Portland , Oregon , United States )
- Jacoby, Daniel ( Cytokinetics , South San Francisco , California , United States )
- Abraham, Theodore ( Univ of California at San Francisco , San Francisco , California , United States )
- Kupfer, Stuart ( Cytokinetics , Highland Park , Illinois , United States )
- Malik, Fady ( Cytokinetics , South San Francisco , California , United States )
- Meng, Lixin ( CYTOKINETICS, INC. , South San Francisco , California , United States )
- Shreay, Sanatan ( Cytokinetics Inc. , Portland , Oregon , United States )
- Wohltman, Amy ( Cytokinetics Inc. , Portland , Oregon , United States )
- Maurer, Mathew ( Columbia University Irving Medical Center , New York , New York , United States )
- Barriales-villa, Roberto ( Complexo Hospitalario Universitario , A Coruna , Spain )
- Claggett, Brian ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Coats, Caroline ( School of Cardiovascular and Metabolic Health, University of Glasgow , Glasgow , United Kingdom )
- Garcia-pavia, Pablo ( Hospital Puerta de Hierro , Majadahonda , Spain )
- Hagege, Albert ( Département de Cardiologie, Assistance Publique Hôpitaux de Paris, Hôpital Européen Georges-Pompidou , Paris , France )
- Januzzi, James ( Massachusetts General Hospital , Wellesley Hills , Massachusetts , United States )
- Kulac, Ian ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
Meeting Info:
Session Info:
More abstracts on this topic:
Sweeney Daniel, Uppal Priya, Jamrozik Thomas, Hutchison Paul
Association between resilience and health status among adults after myocardial infarctionChen Bryan, Liu Olivia, Bartelloni Alexis, Xia Yuhe, Reynolds Harmony, Spruill Tanya, Arabadjian Milla
More abstracts from these authors:
Newlands Chloe, Kulac Ian, Lee Matthew, Maron Martin, Olivotto Iacopo, Owens Anjali, Jacoby Daniel, Heitner Stephen, Kupfer Stuart, Malik Fady, Meng Lixin, Griskowitz Catharine, Wohltman Amy, Malhotra Rajeev, Lewis Gregory, Campain Joseph, Mcginnis Shaina, Giverts Ilya, Moreno Fabely, Landsteiner Isabela, Claggett Brian, Coats Caroline
Effect of Aficamten in Women Compared with Men with Obstructive Hypertrophic CardiomyopathyWang Xiaowen, Nassif Michael, Olivotto Iacopo, Jacoby Daniel, Heitner Stephen, Wohltman Amy, Solomon Scott, Hegde Sheila, Pabon Maria, Abraham Theodore, Barriales-villa Roberto, Claggett Brian, Coats Caroline, Maron Martin, Masri Ahmad, Meder Benjamin