Final ID: 4142627
Cardiac Resident Macrophage Produces Collagen I and Contribute to Vascular Maturation After Myocardial Infarction
Abstract Body (Do not enter title and authors here): Background: Cardiac resident macrophages engage in a wide array of functions such as endocytosis, angiogenesis, and regeneration and are essential to the maintenance of homeostasis in the steady heart. After myocardial infarction, cardiac resident macrophages are highly heterogenous with diverse functions and therapeutic potentials and primarily within the infarct border zone. Cardiac resident macrophages drive postinfarction remodeling by secreting proteins that impart signals to nearby cells expressing their cognate receptors. The full complexity of this intercellular cross-talk that shapes wound healing and functional adaptation after MI remains incompletely understood.
Methods: Single-cell transcriptomes from human and mouse acute myocardial infarction documented CCR2− collagen I+ macrophage expansion. Multiple transgenic mice such us Col1a1 F/F; LysMCre, and Col1a1F/F; Cx3cr1CreER were used to determine the functional significance of collagen I derived from macrophage in MI. Moreover, collagen I expression from macrophages was analyzed by immunohistochemistry and qPCR in the cardiac tissues of patients with ischemic cardiomyopathy and healthy controls.We generated a genetic lineage-tracing system that uses dual recombinases (Cre and Dre) to specifically track collagen I+ macrophages in vivo.
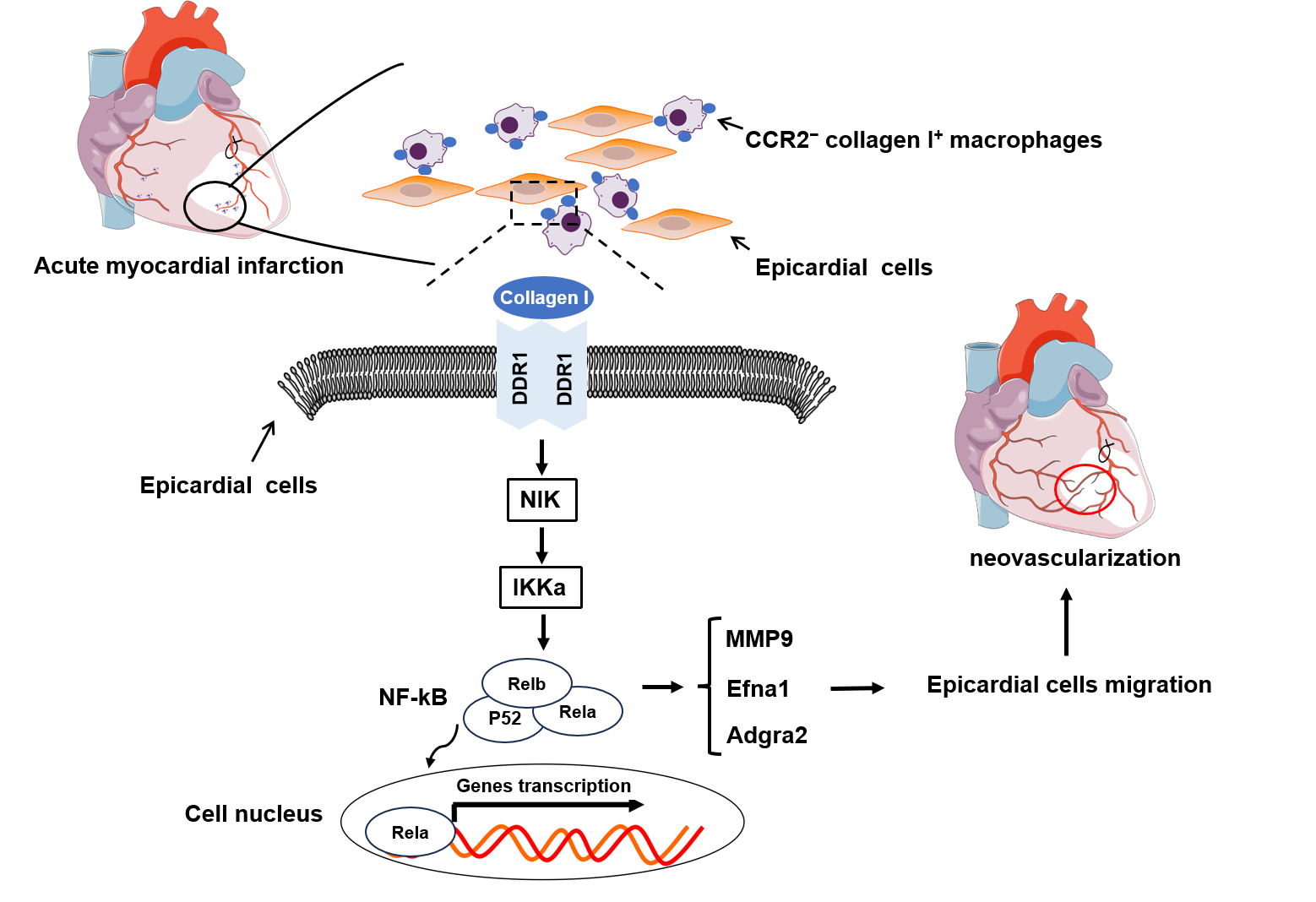
Results:Dual recombinases-mediated genetic system targeted CCR2−MHC-IIlowCol1a1+ macrophages specifically and efficiently. Lineage tracing study revealed accumulation of CCR2−MHC-IIlowCol1a1+ macrophages in the border zone after MI. Macrophage-specific deletion of Col1a1 significantly reduced collagen I production in the border area and resulted in a considerable exacerbation in cardiac function, accompanied with impaired epicardial cell migration and new vessel formation. Collagen I derived from cardiac resident macrophage controls epicardial cell migration through the DDR1–NF-κB–MMP9/Efna1/Adgra2 axis.
Conclusions:In this study, we deciphered how cardiac resident macrophage and epicardial cells cross-talk that shapes wound healing and functional adaptation after myocardial infarction.
Methods: Single-cell transcriptomes from human and mouse acute myocardial infarction documented CCR2− collagen I+ macrophage expansion. Multiple transgenic mice such us Col1a1 F/F; LysMCre, and Col1a1F/F; Cx3cr1CreER were used to determine the functional significance of collagen I derived from macrophage in MI. Moreover, collagen I expression from macrophages was analyzed by immunohistochemistry and qPCR in the cardiac tissues of patients with ischemic cardiomyopathy and healthy controls.We generated a genetic lineage-tracing system that uses dual recombinases (Cre and Dre) to specifically track collagen I+ macrophages in vivo.
Results:Dual recombinases-mediated genetic system targeted CCR2−MHC-IIlowCol1a1+ macrophages specifically and efficiently. Lineage tracing study revealed accumulation of CCR2−MHC-IIlowCol1a1+ macrophages in the border zone after MI. Macrophage-specific deletion of Col1a1 significantly reduced collagen I production in the border area and resulted in a considerable exacerbation in cardiac function, accompanied with impaired epicardial cell migration and new vessel formation. Collagen I derived from cardiac resident macrophage controls epicardial cell migration through the DDR1–NF-κB–MMP9/Efna1/Adgra2 axis.
Conclusions:In this study, we deciphered how cardiac resident macrophage and epicardial cells cross-talk that shapes wound healing and functional adaptation after myocardial infarction.
More abstracts on this topic:
Mitochondrial Proteostasis In Cardiac Fibroblast Activation And Fibrosis
Goyani Shanikumar, Kashyap Shiridhar, Kadam Ashlesha, Shukla Shatakshi, Singh Gunjan, Maxwell Joshua, Jadiya Pooja, Tomar Dhanendra
A Genome-wide CRISPRi Screen Implicates Coronary Artery Disease GWAS Genes as Key Regulators of Adventitial Fibroblast ProliferationJackson William, Zhu Ashley, Gu Wenduo, Berezowitz Alexa, Iyer Meghana, Cheng Paul