Final ID: MDP958
Pulmonary Artery Pressure Monitor is Associated with Improved Outcomes in the Octogenarian-plus Age Group
Abstract Body (Do not enter title and authors here): Background
Pulmonary artery pressure monitoring with an implantable device (PAPM) improves heart failure hospitalizations (HFH) in patients with symptomatic heart failure. This device has been previously validated in clinical trials among participants with a mean age of 69 years and a maximum age of 78 but not in the octogenarian-plus age group (≥80).
Methods
We retrospectively evaluated 277 heart failure (HF) patients who had a PAPM device placed between 1/1/19 and 10/31/22, with at least 1 yr follow up and compared outcomes between patients aged <80yr (n=218) vs aged ≥80yr (n=59). Continuous data were compared with independent samples t-test and categorical data were compared using chi-square or Fisher’s exact test. Multivariable logistic regression was used to further examine post-PAPM outcomes while adjusting for covariates.
Results
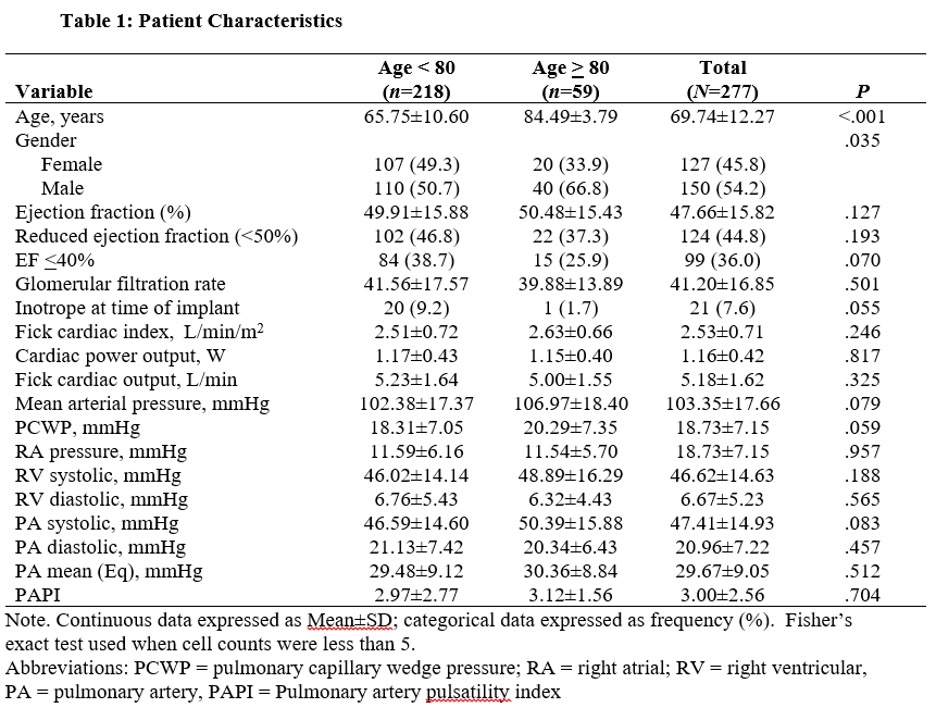
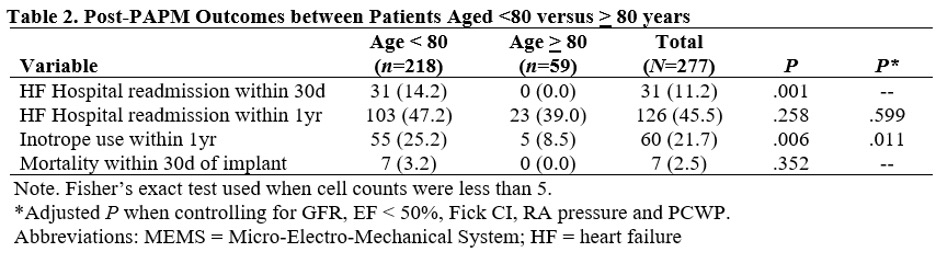
Patients in the <80yr group were aged 65.6 ± 10.6yr and 51% were male & in the ≥80yr group were aged 84.5 ± 3.8yr and 67% were male. Patient characteristics are outlined in table 1. Post-PAPM outcomes between groups are presented in Table 2. HF readmission within 30 days was significantly less in the ≥80yr group compared to the <80yr group (P=.001). HFH at 1yr was not significantly different between groups. Need for inotrope within 1yr was significantly lower in the ≥80yr group compared to the <80yr group (P=.006). Mortality within 30d of PAPM implant was not significantly different between the two groups. Results did not change after adjusting for relevant covariates.
Conclusion
To our knowledge, this study is the first of its kind to show that octogenarian-plus individuals had less 30d HFH and less need for inotropes within 1yr post-PAPM implant compared to those <80yr. There was no difference in mortality between groups. These results indicate that the use a PAPM device in the octogenarian-plus demographic is safe and effective and warrants a prospective clinical trial.
Pulmonary artery pressure monitoring with an implantable device (PAPM) improves heart failure hospitalizations (HFH) in patients with symptomatic heart failure. This device has been previously validated in clinical trials among participants with a mean age of 69 years and a maximum age of 78 but not in the octogenarian-plus age group (≥80).
Methods
We retrospectively evaluated 277 heart failure (HF) patients who had a PAPM device placed between 1/1/19 and 10/31/22, with at least 1 yr follow up and compared outcomes between patients aged <80yr (n=218) vs aged ≥80yr (n=59). Continuous data were compared with independent samples t-test and categorical data were compared using chi-square or Fisher’s exact test. Multivariable logistic regression was used to further examine post-PAPM outcomes while adjusting for covariates.
Results
Patients in the <80yr group were aged 65.6 ± 10.6yr and 51% were male & in the ≥80yr group were aged 84.5 ± 3.8yr and 67% were male. Patient characteristics are outlined in table 1. Post-PAPM outcomes between groups are presented in Table 2. HF readmission within 30 days was significantly less in the ≥80yr group compared to the <80yr group (P=.001). HFH at 1yr was not significantly different between groups. Need for inotrope within 1yr was significantly lower in the ≥80yr group compared to the <80yr group (P=.006). Mortality within 30d of PAPM implant was not significantly different between the two groups. Results did not change after adjusting for relevant covariates.
Conclusion
To our knowledge, this study is the first of its kind to show that octogenarian-plus individuals had less 30d HFH and less need for inotropes within 1yr post-PAPM implant compared to those <80yr. There was no difference in mortality between groups. These results indicate that the use a PAPM device in the octogenarian-plus demographic is safe and effective and warrants a prospective clinical trial.
More abstracts on this topic:
Assessing Prevalence of Cardiac Amyloidosis at the Time of Spinal Laminectomy for Spinal Stenosis
Sul Lidiya, Cotta Claudiu, Nakashima Megan, Steinmetz Michael, Kilpatrick Scott, Reith John, Hanna Mazen, Ives Lauren, Schlenk Richard, Kalfas Iain, Mroz Thomas, Orr Doug, Benzel Edward, Krishnaney Ajit, Pelle Dominic
A novel approach for LV delay evaluation: non-invasive QLV measurement using UHF ECGPoviser Lukas, Stros Petr, Vesela Jana, Curila Karol