Final ID: Su3013
Risk of Suicide, Hair Loss, and Aspiration with Glucagon-like Peptide 1 Receptor Agonists: A Real-World Pharmacovigilance Study from the FAERS database
Abstract Body (Do not enter title and authors here):
Introduction:
With the increasing popularity of glucagon-like peptide 1 receptor agonists (GLP1-RAs), numerous safety concerns arose pertaining to suicide, hair loss, and aspiration risks. We attempted to validate these concerns.
Methods:
We queried the FDA Adverse Event Reporting System (FAERS) database; a post-marketing pharmacovigilance database, from Q4/2003 till Q3/2023 to analyze public reports of these adverse events with GLP1-RAs and other diabetes medications, including sodium-glucose transporter 2 inhibitors (SGLT2is), dipeptidyl peptidase 4 inhibitors (DPP4is), sulfonylureas, metformin, and insulin. OpenVigil 2.1 is an online tool that was utilized to perform disproportionality analysis. A positive signal signifying disproportionate reporting was detected if the proportional reporting ratio (PRR) > 2 and chi-squared (χ2) > 4 for any drug-event pair. The studied medications were arranged in descending order according to the corresponding reporting odds ratio (ROR), which is a measure of the likelihood of reporting a certain event with a certain drug in comparison to all other drugs in the database.
Results:
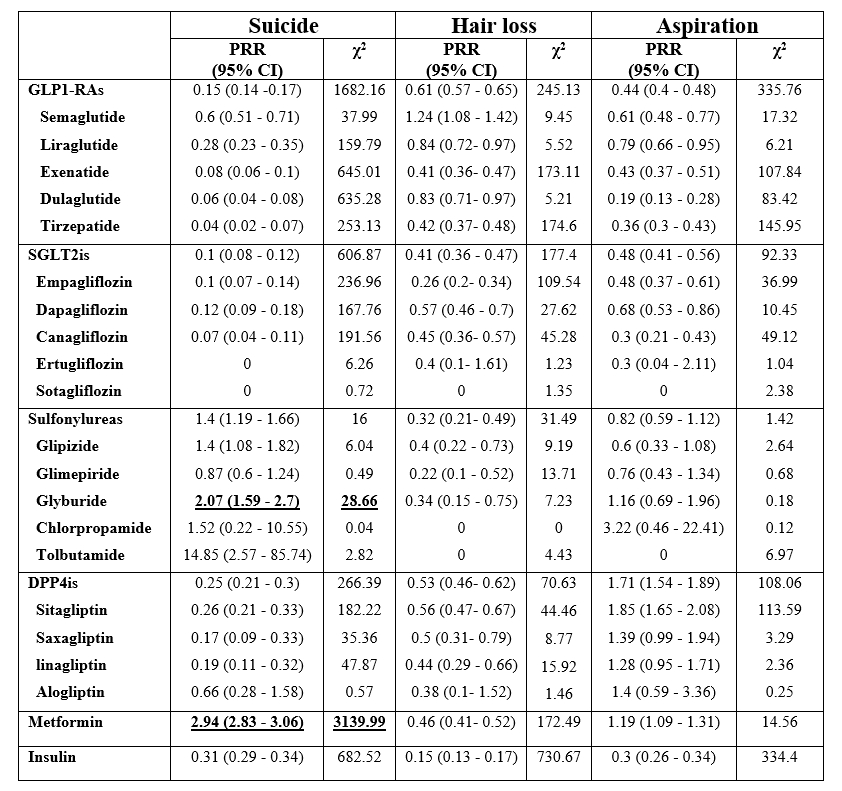
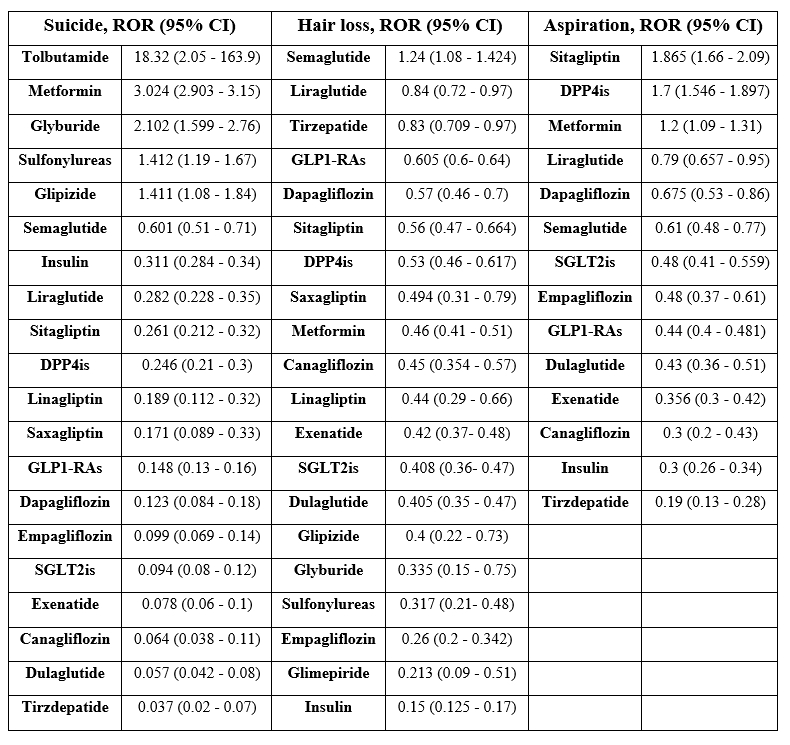
No positive signals were observed between GLP1-RAs and either suicide, hair loss, or aspiration events. Semaglutide [ROR= 0.601 (95% CI 0.51 - 0.71)] and liraglutide [ROR= 0.282 (95% CI 0.228 - 0.35)] had higher suicidal events than DPP4is and SGLT2is. GLP1-RAs were the most reported class with hair loss [ROR= 0.605 (95% CI 0.6 - 0.64)], and semaglutide, liraglutide, and dulaglutide were the three leading medications. GLP1-RAs ranked lower with aspiration events, which were led by sitagliptin and DPP4i as a group. Only metformin and glyburide generated positive signals with suicide risk.
Conclusion:
GLP1-RAs exhibit higher reporting of suicide, hair loss, and aspiration events when compared to several other antidiabetic medications, despite not meeting the criteria for positive signals yet. This warrants intensive monitoring and reporting.
Introduction:
With the increasing popularity of glucagon-like peptide 1 receptor agonists (GLP1-RAs), numerous safety concerns arose pertaining to suicide, hair loss, and aspiration risks. We attempted to validate these concerns.
Methods:
We queried the FDA Adverse Event Reporting System (FAERS) database; a post-marketing pharmacovigilance database, from Q4/2003 till Q3/2023 to analyze public reports of these adverse events with GLP1-RAs and other diabetes medications, including sodium-glucose transporter 2 inhibitors (SGLT2is), dipeptidyl peptidase 4 inhibitors (DPP4is), sulfonylureas, metformin, and insulin. OpenVigil 2.1 is an online tool that was utilized to perform disproportionality analysis. A positive signal signifying disproportionate reporting was detected if the proportional reporting ratio (PRR) > 2 and chi-squared (χ2) > 4 for any drug-event pair. The studied medications were arranged in descending order according to the corresponding reporting odds ratio (ROR), which is a measure of the likelihood of reporting a certain event with a certain drug in comparison to all other drugs in the database.
Results:
No positive signals were observed between GLP1-RAs and either suicide, hair loss, or aspiration events. Semaglutide [ROR= 0.601 (95% CI 0.51 - 0.71)] and liraglutide [ROR= 0.282 (95% CI 0.228 - 0.35)] had higher suicidal events than DPP4is and SGLT2is. GLP1-RAs were the most reported class with hair loss [ROR= 0.605 (95% CI 0.6 - 0.64)], and semaglutide, liraglutide, and dulaglutide were the three leading medications. GLP1-RAs ranked lower with aspiration events, which were led by sitagliptin and DPP4i as a group. Only metformin and glyburide generated positive signals with suicide risk.
Conclusion:
GLP1-RAs exhibit higher reporting of suicide, hair loss, and aspiration events when compared to several other antidiabetic medications, despite not meeting the criteria for positive signals yet. This warrants intensive monitoring and reporting.
More abstracts on this topic:
Age-standardized trends in Incidence Rates of Noncommunicable diseases among Adults Aged 30 to 79 in Senegal from 2000 to 2019
Gaye Ngone, Ka Mame, Kyem Damaris, Jobe Modou, Sattler Elisabeth, Gary-webb Tiffany, Gaye Bamba
Artificial Intelligence-Enabled Electrocardiography For The Prediction of Future Type 2 Diabetes MellitusPastika Libor, Peters Nicholas, Kramer Daniel, Waks Jonathan, Sau Arunashis, Ng Fu Siong, Patlatzoglou Konstantinos, Sieliwonczyk Ewa, Barker Joseph, Zeidaabadi Boroumand, Mcgurk Kathryn, Khan Sadia, Mandic Danilo, Ware James