Final ID: Sa2067
Quality of Life Outcomes after the Dual Epicardial & Endocardial Procedure Approach for Treatment of Non-Paroxysmal Atrial Fibrillation
Goal: To evaluate QOL changes from baseline to 12-months post-HA+LAAE in the DEEP trial.
Methods: DEEP is a 17-site prospective, international, single arm trial. Key eligibility: adults with non-paroxysmal AF, left atrial diameter ≤5.5cm, failed class I/III anti-arrhythmic drugs, and ≤2 prior failed CA. Stage 1 was thoracoscopic radiofrequency epicardial ablation to isolate the pulmonary veins and left atrial posterior wall, and LAAE. Stage 2 was endocardial mapping and touchup ablation. QOL was measured via the Atrial Fibrillation Effect on Quality-of-Life (AFEQT) questionnaire at baseline and 12-months post-HA+LAAE. AFEQT yields scores from 0 to 100 (0 as complete disability and 100 as no disability) and includes an overall and four subscale scores. A 5-point AFEQT change is clinically important. Mean differences with 95% CIs were calculated; p-values are from paired t-tests.
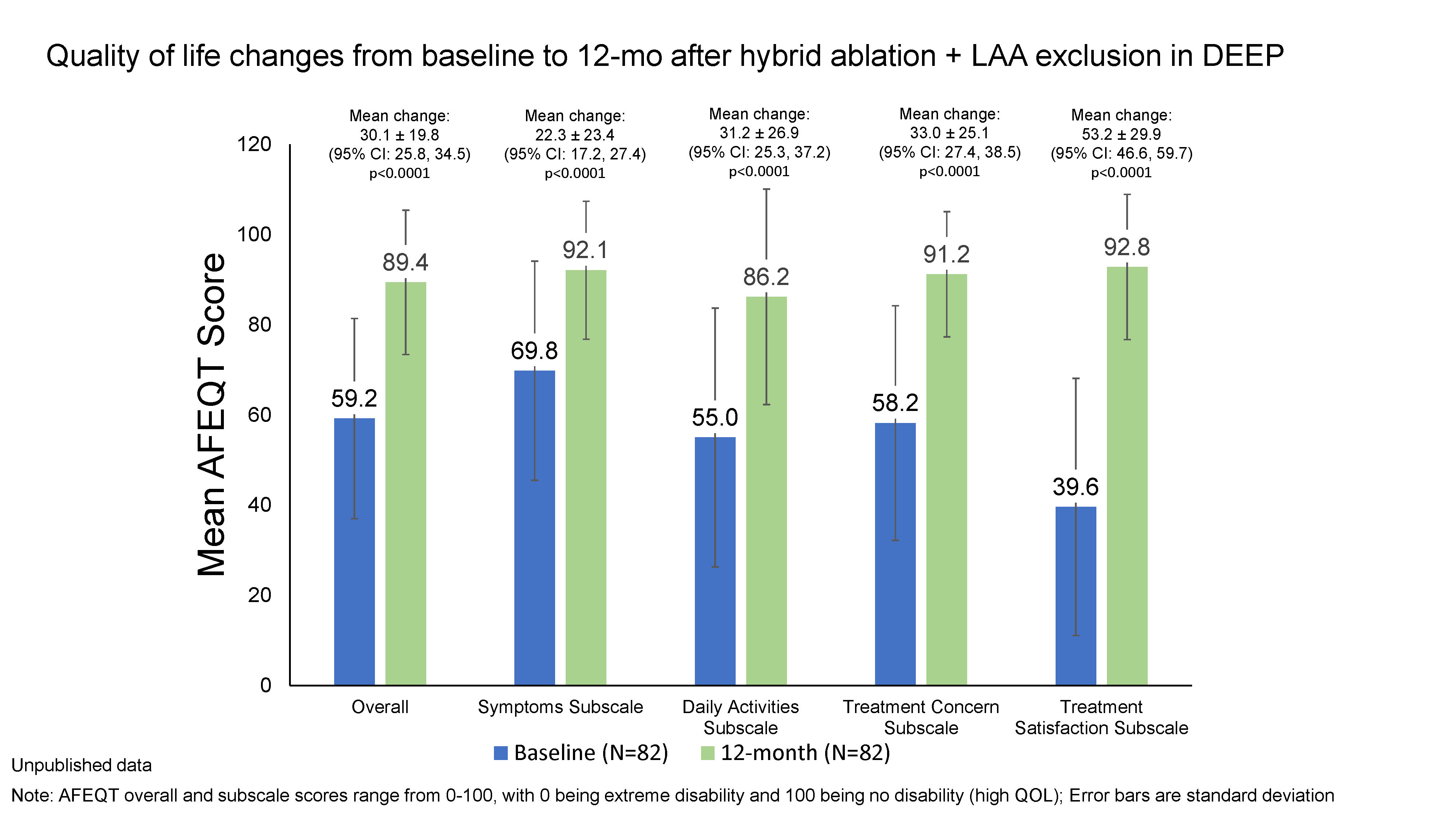
Results: Of 85 patients, 82 completed both baseline and 12-month AFEQT. The mean change in overall score was 30.1 ± 19.8 (95% CI: 25.8-34.5; median 29.5, min, max: -15.4, 78.2; p<0.0001) (Figure). The greatest improvement was in the Treatment Satisfaction subscale with a mean score of 53.2 ± 29.9 (95% CI: 46.6-59.7; median 58.3, min, max: 0.0, 100.0; p<0.0001), followed by Treatment Concern, Daily Activities, and Symptoms subscale scores. All mean improvements from baseline exceeded a clinically meaningful 5-point change.
Conclusion: In the DEEP trial, HA+LAAE resulted in patient-reported QOL improvements at 12-months post-procedure.
- Khoynezhad, Ali ( MemorialCare Long Beach Med Center , Manhattan Beach , California , United States )
- De Groot, Joris ( Amsterdam University Medical Center , Amsterdam , Netherlands )
- Driessen, Antoine ( Amsterdam University Medical Center , Amsterdam , Netherlands )
- Lee, Mark ( Long Beach Medical Center , Long Beach , California , United States )
- Hoff, Steven ( Baptist Health Medical Group , Miami , Florida , United States )
- Bello, David ( Orlando Health-Heart Institute , Orlando , Florida , United States )
- Dunnington, Gansevoort ( St. Helena Hospital , Saint Helena , California , United States )
- Eisenberg, Susan ( St. Helena Hospital , Saint Helena , California , United States )
- Vloka, Margot ( St. Alphonsus Regional Medical Center , Boise , Idaho , United States )
- Taylor, Benedict ( St. Alphonsus Regional Medical Center , Boise , Idaho , United States )
- Jones, Stephen ( St. Alphonsus Regional Medical Center , Boise , Idaho , United States )
- Kasirajan, Vigneshwar ( WEST HOSPTIAL , Richmond , Virginia , United States )
- Philpott, Jonathan ( Hawaii Permanente Medical Group , Honolulu , Hawaii , United States )
- Beaver, Thomas ( UNIVERSITY FLORIDA , Gainesville , Florida , United States )
- Miles, William ( UNIV OF FLORIDA , Gainesville , Florida , United States )
- Khan, Junaid ( Alta Bates Summit Medical Center , Oakland , California , United States )
- Kang, Steven ( Alta Bates Summit Medical Center , Oakland , California , United States )
- Gandhi, Gaurang ( TriHealth Heart Institute , Cincinnati , Ohio , United States )
- Okum, Eric ( TriHealth Heart Institute , Cincinnati , Ohio , United States )
- Badhwar, Nitesh ( Stanford University , Palo Alto , California , United States )
- Baykaner, Tina ( Stanford University , Palo Alto , California , United States )
- Lee, Anson ( STANFORD UNIVERSITY , Stanford , California , United States )
- La Meir, Mark ( University Hospital Brussels , Brussels , Belgium )
- Vesco, Paul ( Sarasota Memorial Hospital , Sarasota , Florida , United States )
- Smith, J Michael ( The Christ Hospital , Cincinnati , Ohio , United States )
- Gaynor, Sydney ( AtriCure , Mason , Ohio , United States )
- Frazier, Ken ( AtriCure , Mason , Ohio , United States )
- Ellembogen, Kenneth ( Virginia Commonwealth University , Richmond , Virginia , United States )
- De Asmundis, Carlo ( UZ Brussel VUB , Brussels , Belgium )
- Koneru, Jayanthi ( Virginia Commonwealth University , Richmond , Virginia , United States )
- Johnkoski, John ( Aspirus Wausau Hospital , Wausau , Wisconsin , United States )
- Rist, Kevin ( Aspirus Wausau Hospital , Wausau , Wisconsin , United States )
- Mumtaz, Mubashir ( UPMC , Harrisburg , Pennsylvania , United States )
- Link, Michael ( UPMC , Harrisburg , Pennsylvania , United States )
Meeting Info:
Session Info:
Atrial Fibrillation Ablation: Heart Failure, Myopathies and More
Saturday, 11/16/2024 , 10:30AM - 11:30AM
Abstract Poster Session
More abstracts on this topic:
Futela Pragyat, Poddar Aastha, Kowlgi Gurukripa
A comparison of the efficacy of initial high energy versus initial low energy biphasic shocks for cardioversion of atrial fibrillation and atrial flutter – a real-life experienceAlampoondi Venkataramanan Sai Vikram, Vunnam Ramarao, Voruganti Dinesh, Tsai Shane