Final ID: Sa3071
Shifting trends and study characteristics associated with randomization, blinding, and data monitoring committee oversight of cardiovascular trials: analysis of ClinicalTrials.gov listings from 2000 to 2023
Abstract Body (Do not enter title and authors here):
Background: In response to concerns regarding insufficient transparency and selective reporting, the International Committee of Medical Journal Editors (ICMJE) issued a statement in 2004 mandating the registration of all clinical trials in a publicly accessible repository. Its impact on the trend of cardiovascular (CV) trial has not been comprehensively examined. The relationship between trial characteristics and designs aimed at enhancing methodological rigor and scientific validity, such as the implementation of randomization, blinding, and a data monitoring committee (DMC), remains unclear.
Methods: The analysis included studies that: (1) were registered in ClinicalTrials.gov between 2000 and 2023; (2) involved ≥1 intervention; and (3) investigated ≥1 CV condition. Interrupted time-series analysis was performed to assess the impact of the ICMJE policy on the annual trend of trial registration. Multivariable logistic regression was conducted to assess the association between study characteristics and randomization, blinding, or DMC oversight.
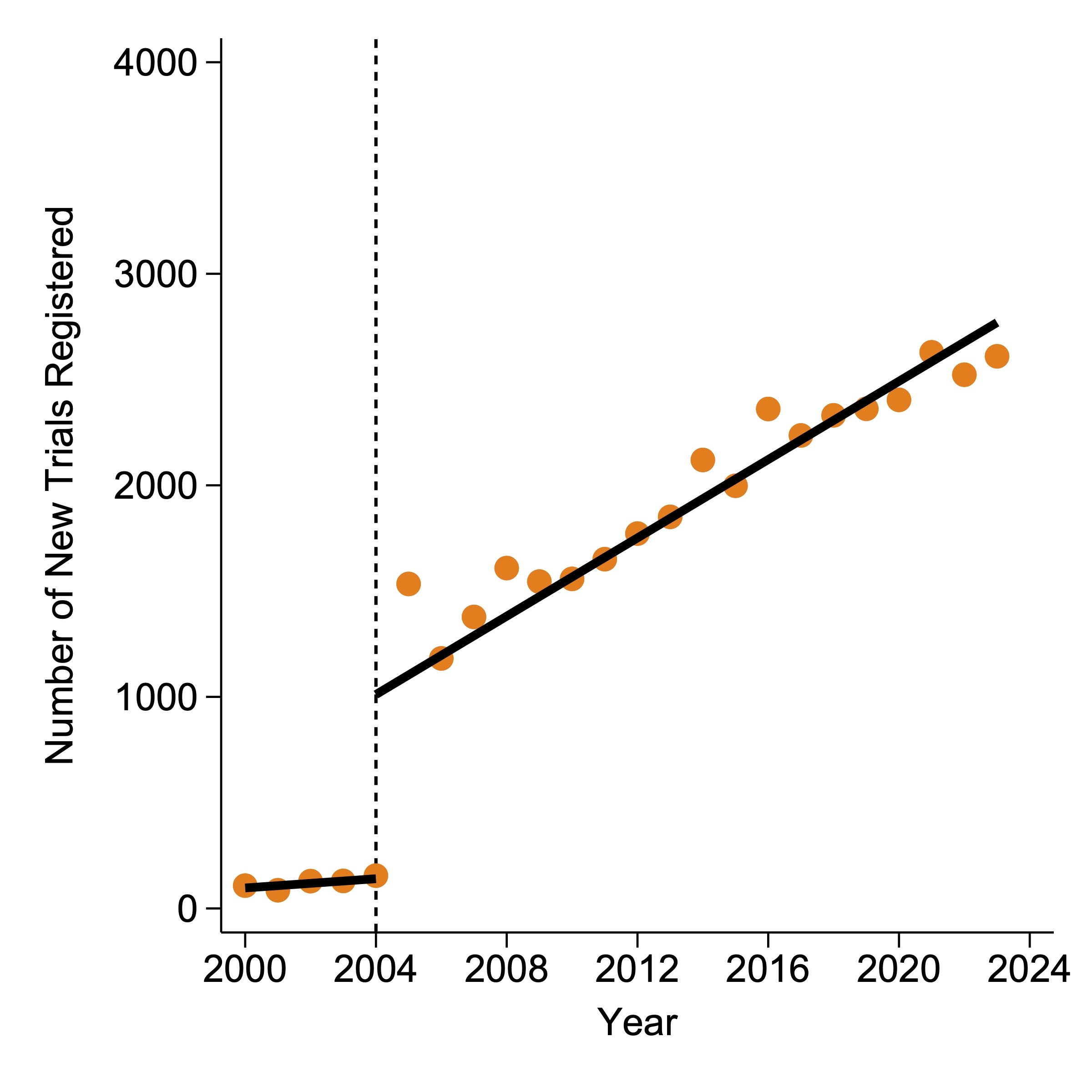
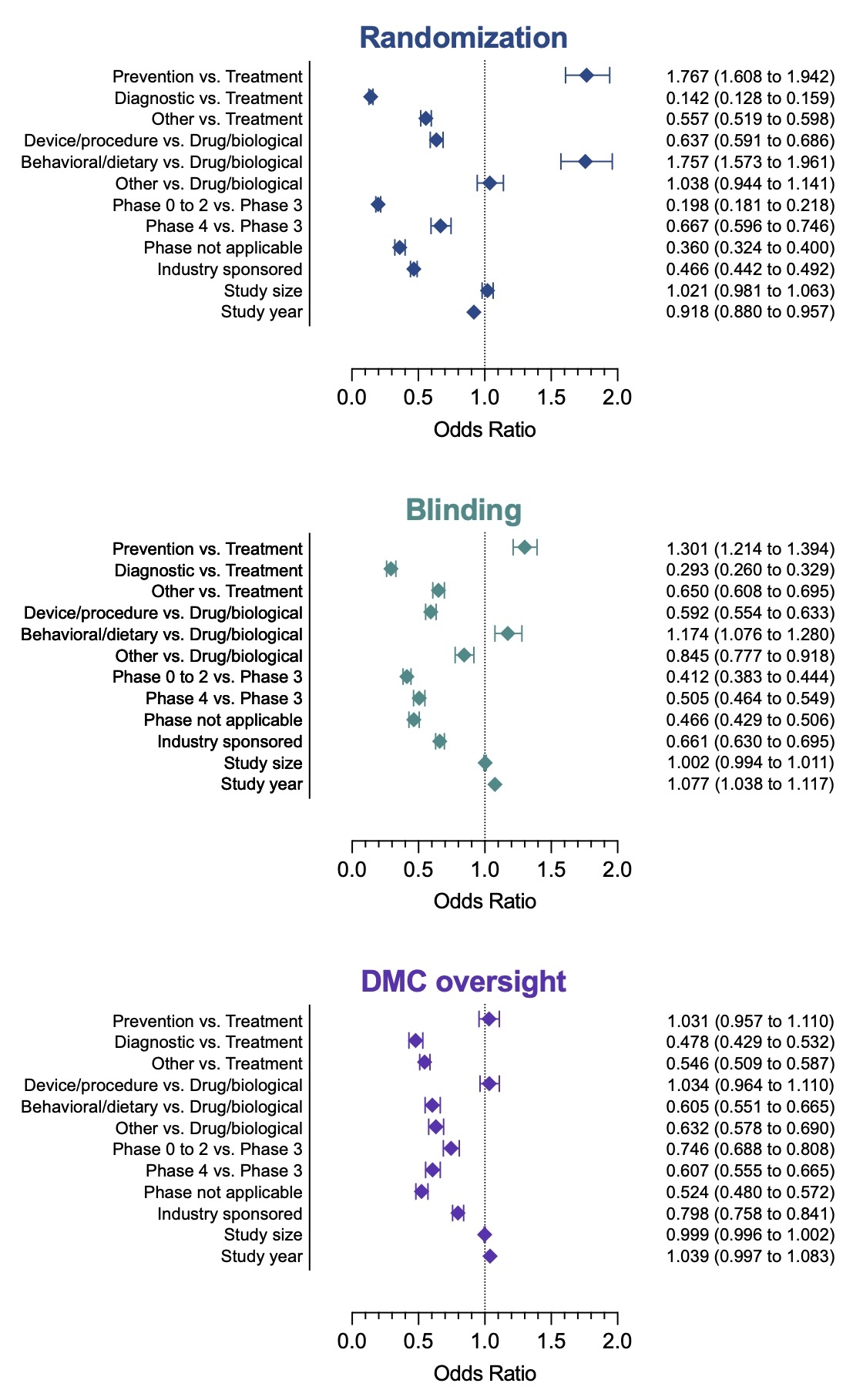
Results: A total of 38,262 CV trials were identified from 473,593 records. Prior to the implementation of ICMJE policy, the number of new CV trials increased by 10.9 (95% confidence interval [CI]: 1.5 to 20.3) every year. Following the implementation, the ascending trend intensified significantly (p<0.001), with a rate of 92.6 (95% CI: 60.6 to 124.5) new CV trials per year (Figure 1). Randomization and blinding were more commonly adopted in preventive trials, trials involving behavioral or dietary interventions, and phase 3 trials (Figure 2). In contrast, diagnostic trials and trials related to devices or procedures were less likely to include randomization or blinding in their design. More recent trials were less likely to implement randomization but more likely to implement blinding than earlier trials. DMC oversight was less prevalent in diagnostic trials, trials involving behavioral or dietary interventions, and non-phase 3 trials. Trials supported by the industry were less inclined to employ randomization, blinding, or DMC oversight. Study size was not correlated with any of these designs.
Conclusion: The upward trend of CV trial registration augmented following the implementation of the ICMJE policy. Implementation of randomization, blinding, and DMC oversight was associated with study purpose, intervention, phase, funding source, and start year, with no discernible variation by study size.
Background: In response to concerns regarding insufficient transparency and selective reporting, the International Committee of Medical Journal Editors (ICMJE) issued a statement in 2004 mandating the registration of all clinical trials in a publicly accessible repository. Its impact on the trend of cardiovascular (CV) trial has not been comprehensively examined. The relationship between trial characteristics and designs aimed at enhancing methodological rigor and scientific validity, such as the implementation of randomization, blinding, and a data monitoring committee (DMC), remains unclear.
Methods: The analysis included studies that: (1) were registered in ClinicalTrials.gov between 2000 and 2023; (2) involved ≥1 intervention; and (3) investigated ≥1 CV condition. Interrupted time-series analysis was performed to assess the impact of the ICMJE policy on the annual trend of trial registration. Multivariable logistic regression was conducted to assess the association between study characteristics and randomization, blinding, or DMC oversight.
Results: A total of 38,262 CV trials were identified from 473,593 records. Prior to the implementation of ICMJE policy, the number of new CV trials increased by 10.9 (95% confidence interval [CI]: 1.5 to 20.3) every year. Following the implementation, the ascending trend intensified significantly (p<0.001), with a rate of 92.6 (95% CI: 60.6 to 124.5) new CV trials per year (Figure 1). Randomization and blinding were more commonly adopted in preventive trials, trials involving behavioral or dietary interventions, and phase 3 trials (Figure 2). In contrast, diagnostic trials and trials related to devices or procedures were less likely to include randomization or blinding in their design. More recent trials were less likely to implement randomization but more likely to implement blinding than earlier trials. DMC oversight was less prevalent in diagnostic trials, trials involving behavioral or dietary interventions, and non-phase 3 trials. Trials supported by the industry were less inclined to employ randomization, blinding, or DMC oversight. Study size was not correlated with any of these designs.
Conclusion: The upward trend of CV trial registration augmented following the implementation of the ICMJE policy. Implementation of randomization, blinding, and DMC oversight was associated with study purpose, intervention, phase, funding source, and start year, with no discernible variation by study size.
More abstracts on this topic:
3-Minute Heart Health App: A Feasibility Study
Abdulkarim Iya, Metzger Joseph, Stovitz Steven, Van't Hof Jeremy
A Randomized Clinical Trial for Asymptomatic Elevated Blood Pressure in Patients Discharged from Emergency DepartmentPrendergast Heather, Khosla Shaveta, Kitsiou Spyros, Petzel Gimbar Renee, Freels Sally, Sanders Anissa, Daviglus Martha, Carter Barry, Del Rios Marina, Heinert Sara