Final ID: Su1032
Uncovering the Mechanisms by Which SGLT-2 Inhibitors Improve Cardiovascular Outcomes: A Proteomic Analysis
Abstract Body (Do not enter title and authors here):
Introduction: Sodium-glucose cotransporter-2 (SGLT-2) inhibitors have been shown to improve cardiovascular outcomes, as evidenced by their inclusion as a preferred treatment option in the ACC/AHA/HFSA guidelines for heart failure management. However, the mechanisms underlying these benefits are not well-understood. The goal of this study is to address this gap through high-sensitivity proteomic analyses to characterize the specific molecular pathways affected by SGLT-2 inhibitor canagliflozin (CAN) administration in a large animal model of coronary artery disease and metabolic syndrome.
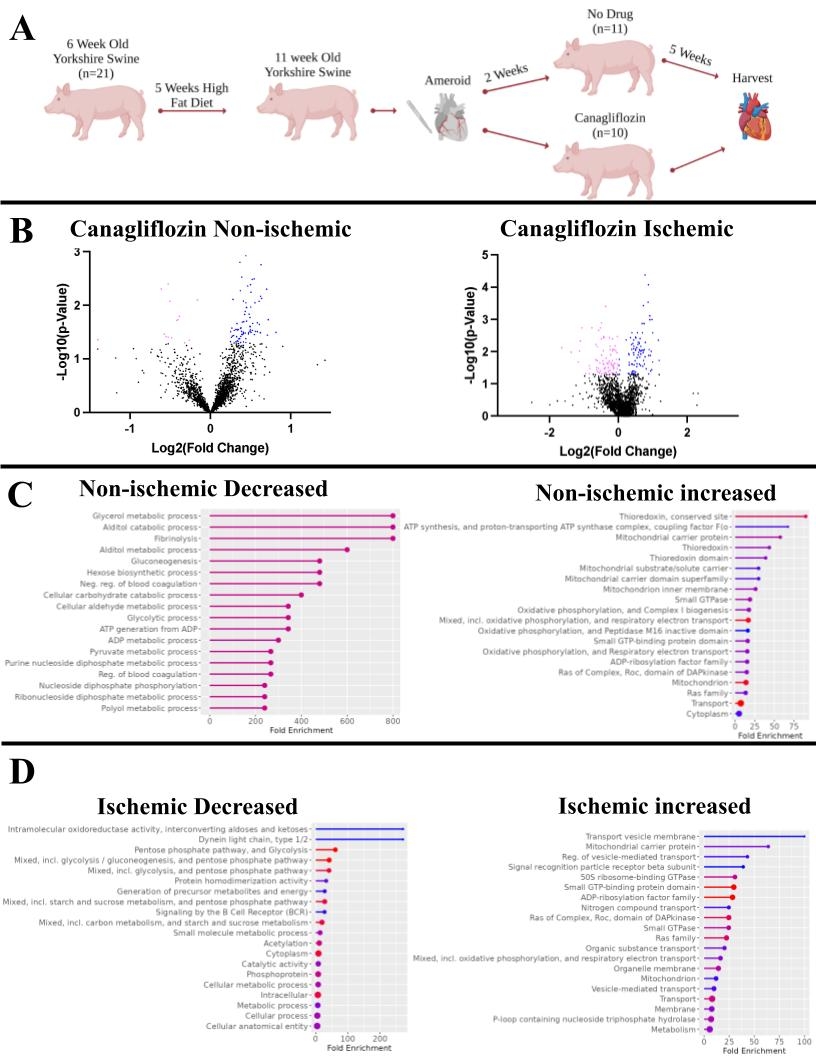
Methods: Twenty-one six-week-old Yorkshire swine were fed a high-fat diet for induction of metabolic syndrome, after which chronic myocardial ischemia was induced by placing an ameroid constrictor around the left circumflex coronary artery. Two weeks later, the swine either received no drug (CON n=11) or 300mg of CAN daily (n=10). Treatment lasted for five weeks, after which myocardial tissue from both ischemic and non-ischemic regions was collected and sent for proteomic analysis.
Results: Proteomic analysis identified a total of 2855 common proteins between ischemic and nonischemic CAN groups. The CAN ischemic group had 124 upregulated and 111 downregulated proteins as compared to CON; pathway analysis applied to this data yielded upregulation of oxidative phosphorylation and respiratory electron transport, as well as downregulation of glycolysis and the pentose phosphate pathway. The CAN nonischemic group had 121 upregulated and 25 down regulated proteins as compared to the CON group. Pathway analysis showed that of the up- and downregulated proteins, significant alterations included upregulation of oxidative phosphorylation and downregulation of gluconeogenesis and glycolysis.
Conclusion: CAN treatment in both ischemic and nonischemic myocardial tissue upregulates oxidative phosphorylation and downregulates glycolysis in a clinically relevant large animal model. These changes ameliorate metabolic derangement in the ischemic myocardium and provide further mechanistic insights into the molecular function of SGLT-2 inhibitors in ischemic heart disease.
Introduction: Sodium-glucose cotransporter-2 (SGLT-2) inhibitors have been shown to improve cardiovascular outcomes, as evidenced by their inclusion as a preferred treatment option in the ACC/AHA/HFSA guidelines for heart failure management. However, the mechanisms underlying these benefits are not well-understood. The goal of this study is to address this gap through high-sensitivity proteomic analyses to characterize the specific molecular pathways affected by SGLT-2 inhibitor canagliflozin (CAN) administration in a large animal model of coronary artery disease and metabolic syndrome.
Methods: Twenty-one six-week-old Yorkshire swine were fed a high-fat diet for induction of metabolic syndrome, after which chronic myocardial ischemia was induced by placing an ameroid constrictor around the left circumflex coronary artery. Two weeks later, the swine either received no drug (CON n=11) or 300mg of CAN daily (n=10). Treatment lasted for five weeks, after which myocardial tissue from both ischemic and non-ischemic regions was collected and sent for proteomic analysis.
Results: Proteomic analysis identified a total of 2855 common proteins between ischemic and nonischemic CAN groups. The CAN ischemic group had 124 upregulated and 111 downregulated proteins as compared to CON; pathway analysis applied to this data yielded upregulation of oxidative phosphorylation and respiratory electron transport, as well as downregulation of glycolysis and the pentose phosphate pathway. The CAN nonischemic group had 121 upregulated and 25 down regulated proteins as compared to the CON group. Pathway analysis showed that of the up- and downregulated proteins, significant alterations included upregulation of oxidative phosphorylation and downregulation of gluconeogenesis and glycolysis.
Conclusion: CAN treatment in both ischemic and nonischemic myocardial tissue upregulates oxidative phosphorylation and downregulates glycolysis in a clinically relevant large animal model. These changes ameliorate metabolic derangement in the ischemic myocardium and provide further mechanistic insights into the molecular function of SGLT-2 inhibitors in ischemic heart disease.
More abstracts on this topic:
Apolipoprotein A-I Proteoforms in Large HDL are Associated with Incident Myocardial Infarction: Observations from Dallas Heart Study
Gangwar Anamika, Des Soye Benjamin, Saldanha Suzanne, Jaiswal Shailesh, Melchior John, Mcdermott Jason, Wilkins John, Rohatgi Anand
Age-Related Impairment of Mitochondrial Protein Turnover Exacerbates Pathogenesis of Heart Failure with Preserved Ejection Fraction in Old MiceKobak Kamil, Zarzycka Weronika, King Catherine, Borowik Agnieszka, Peelor Frederick, Kinter Michael, Miller Benjamin, Chiao Ying Ann