Final ID: MDP78
The Perinexal Nanodomain Within Intercalated Discs Between Isolated Cardiomyocyte Pairs Modulates Sodium Currents
Abstract Body (Do not enter title and authors here): Background: Ephaptic coupling (EpC), a form of intercellular communication between excitable cells, has been proposed to occur at the perinexus, a sodium channel-rich nanodomain within the intercalated disc (ID). Previous studies suggest that widening the perinexus can influence EpC by altering sodium ion availability, which in turn determines perinexal sodium currents (INa). However, there is a lack of direct evidence for a causal relationship between the perinexus and ephaptic coupling. This study aims to develop a novel approach to directly measure the effect of perinexal expansion on INa. We hypothesize that widening the perinexus increases INa in cardiomyocytes that maintain intact IDs.
Methods: We selected pairs of mouse cardiomyocytes connected through IDs, preserving intact perinexi. Initially, the feasibility of measuring the whole-cell INa from the entire cell pair was investigated using single patch clamp. If feasible, widening the perinexus should increase the whole-cell INa by enhancing perinexal INa. Isolated single cardiomyocytes and cardiomyocyte pairs from ventricles were used for whole-cell INa recordings. A de-adhesion peptide named βadp1, previously demonstrated to widen the perinexus, was used.
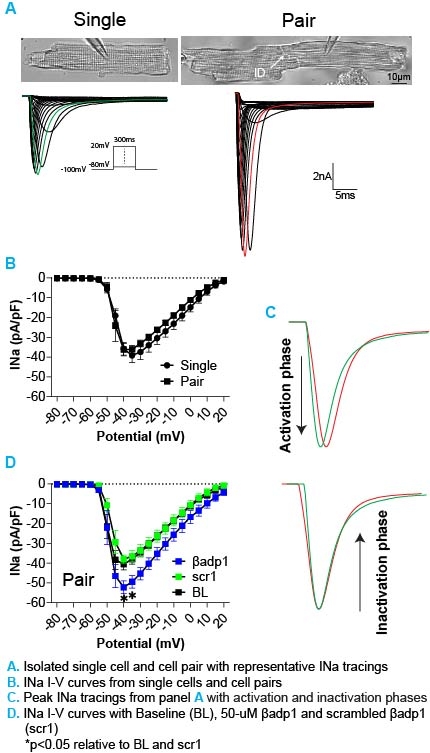
Results: We found that the whole-cell INa in cell pairs can be stably measured, showing no differences in INa density compared to single cells (panels A&B in Figure). Notably, the duration of the INa tracing in cell pairs is short relative to single cells primarily due to shorter inactivation phase in cell pairs (panel C), suggesting a lack of available sodium ions in the ID caused by sodium channel activation. It was also confirmed that βadp1 does not affect INa in single cells, consistent with previous reports. Importantly, we demonstrated that βadp1 significantly increased INa (panel D), likely due to faster sodium influx during sodium channel activation phase.
Conclusions: The cell pair model is a feasible method for measuring whole-cell INa with single patch clamp, and widening the perinexus increases INa in this model, which supports the hypothesis that EpC is occurring between the cells. Our findings may offer a new approach to study the impact of structural changes in IDs on ionic currents.
Methods: We selected pairs of mouse cardiomyocytes connected through IDs, preserving intact perinexi. Initially, the feasibility of measuring the whole-cell INa from the entire cell pair was investigated using single patch clamp. If feasible, widening the perinexus should increase the whole-cell INa by enhancing perinexal INa. Isolated single cardiomyocytes and cardiomyocyte pairs from ventricles were used for whole-cell INa recordings. A de-adhesion peptide named βadp1, previously demonstrated to widen the perinexus, was used.
Results: We found that the whole-cell INa in cell pairs can be stably measured, showing no differences in INa density compared to single cells (panels A&B in Figure). Notably, the duration of the INa tracing in cell pairs is short relative to single cells primarily due to shorter inactivation phase in cell pairs (panel C), suggesting a lack of available sodium ions in the ID caused by sodium channel activation. It was also confirmed that βadp1 does not affect INa in single cells, consistent with previous reports. Importantly, we demonstrated that βadp1 significantly increased INa (panel D), likely due to faster sodium influx during sodium channel activation phase.
Conclusions: The cell pair model is a feasible method for measuring whole-cell INa with single patch clamp, and widening the perinexus increases INa in this model, which supports the hypothesis that EpC is occurring between the cells. Our findings may offer a new approach to study the impact of structural changes in IDs on ionic currents.
More abstracts on this topic:
Direct effect of sodium-glucose cotransporter 2 (SGLT2) inhibitors on cardiomyocyte function as a potential therapy for Phospholamban cardiomyopathy
Hnatiuk Anna, Li Alexander, Staudt David, Serrano Ricardo, Mercola Mark
Effect of Sodium-Glucose Cotransporter-2 Inhibitors on Atrial Fibrillation Recurrence Post-Ablation: A Systematic Review and Meta-AnalysisNugooru Sudeep, Watson Robert, Ginnaram Shravya, Janga Chaitra, Sevella Prerana, Muhammadzai Hamza, Shah Shreeja, Sabri Muhammad, Alhassani Zaineb, Lee Tyler