Final ID: Sa1081
Endothelial-Mesenchymal Transition Mediated by Mechanical Stress Prompts Atrial Fibrogenesis
Abstract Body (Do not enter title and authors here): Background: Atrial fibrosis is crucial in developing atrial fibrillation (AF). Elevated atrial pressure may significantly mediate atrial fibrosis, yet its underlying mechanisms remain unclear.
Methods: Patients with AF who underwent radiofrequency ablation were recruited. Clinical data, including high-density mapping and imaging information, was analyzed. Multivariate regression analysis was performed to identify risk factors for low-voltage areas in the atrium. The CS-CREM mouse model, an autonomic AF model, was previously developed by our research group. Millar pressure catheters were used to measure left ventricular, right ventricular, and right atrial pressures in CS-CREM mice. Single-nucleus sequencing was employed to map the single-cell transcriptomes of atrial samples in CS-CREM and wild-type mice at different disease stages. Human primary atrial endocardial endothelial cells (ACCE) and HUVEC cell lines were subjected to mechanical stretch using the Flexcell tension system, followed by in vitro validation experiments. Mg101, a calpain inhibitor, was administered to CS-CREM mice for in vivo validation experiments.
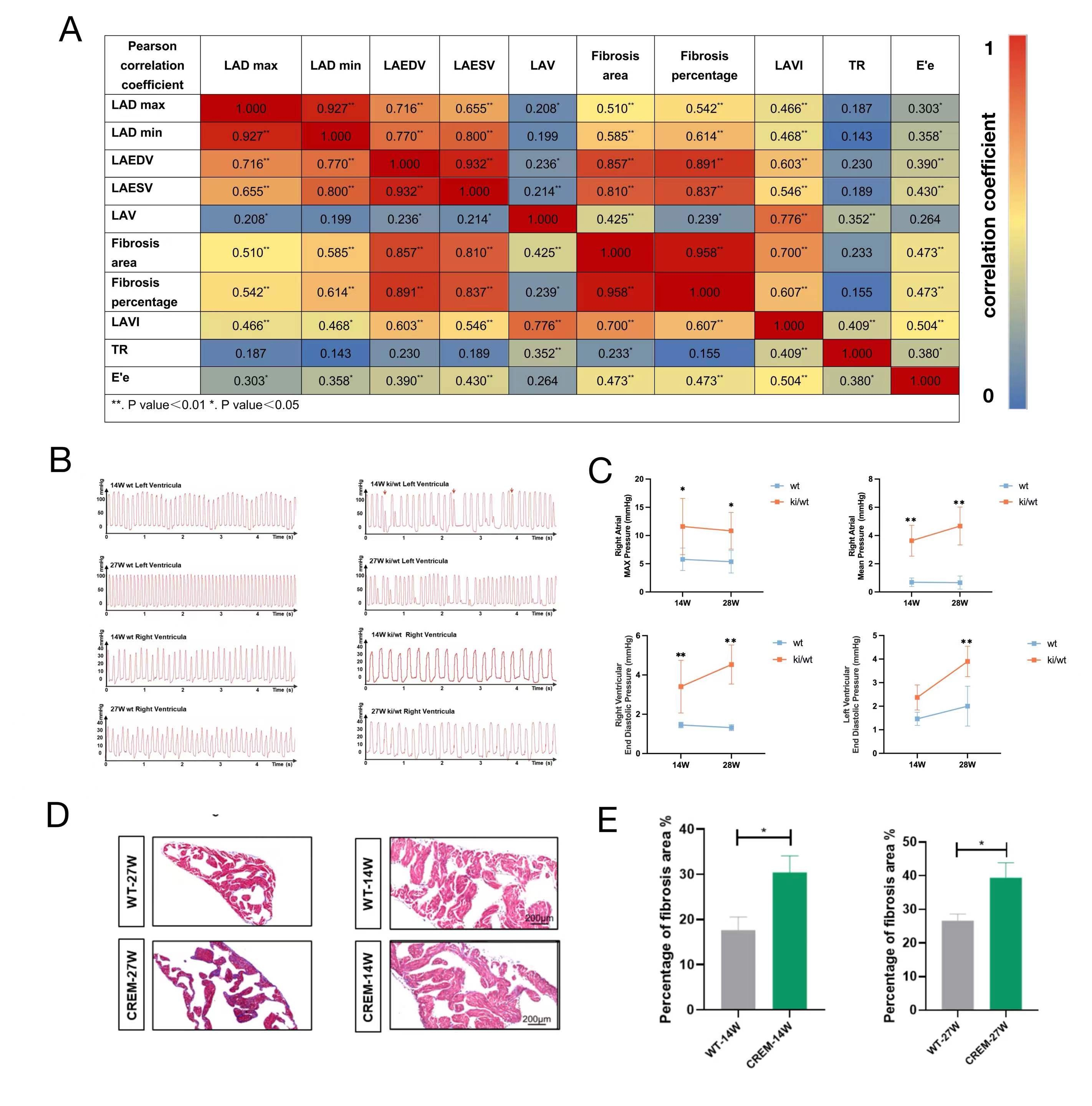
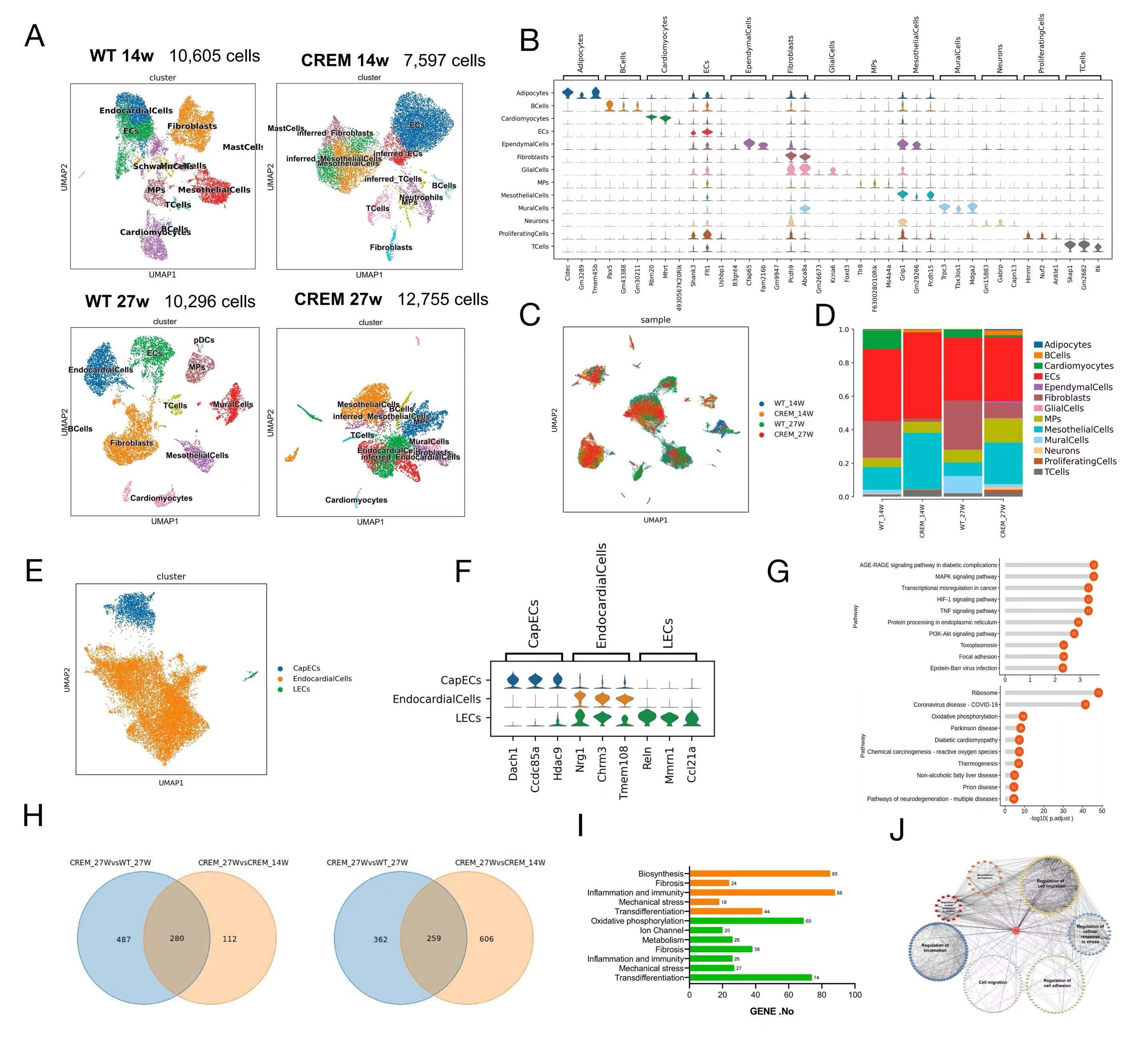
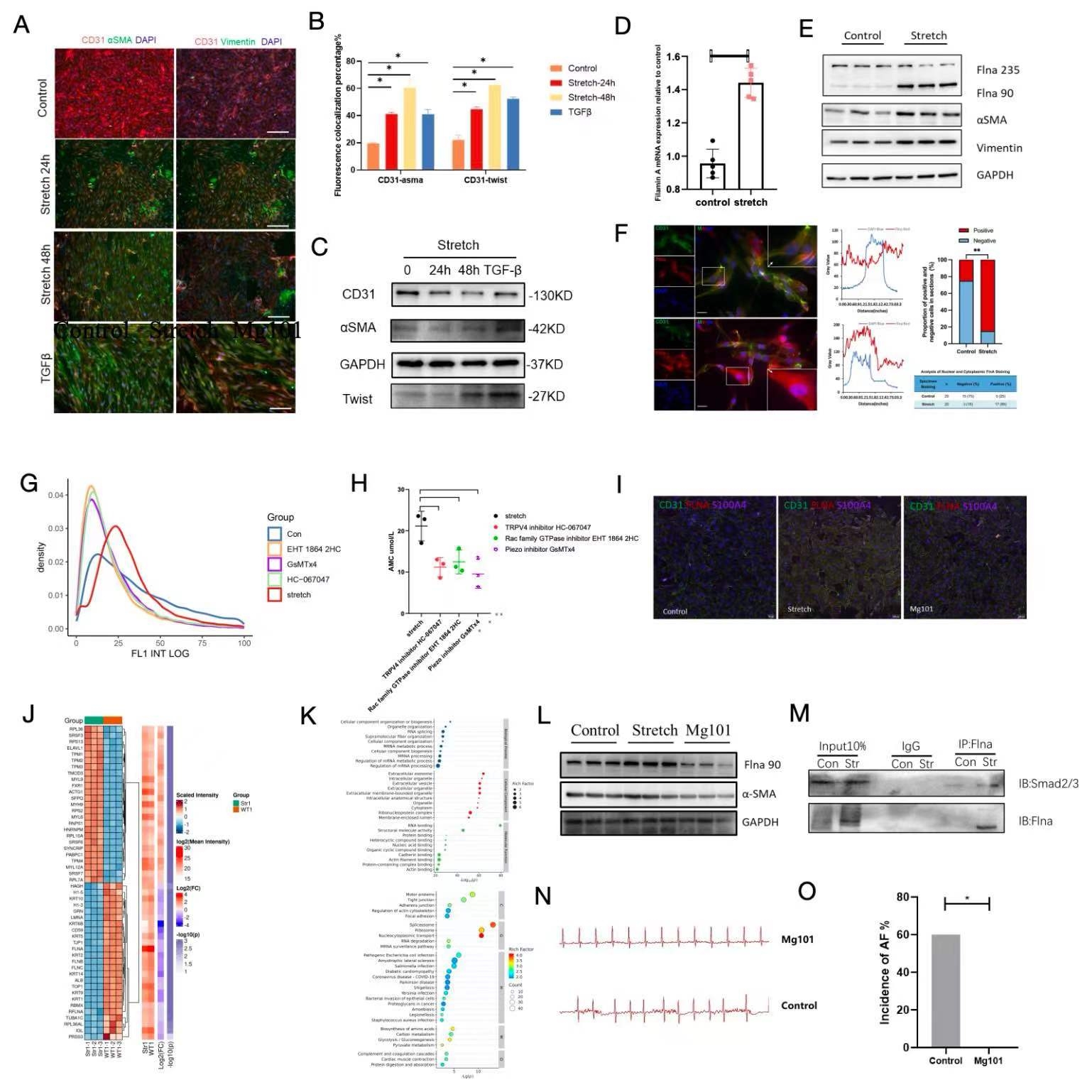
Results: Elevated atrial pressure in AF patients was identified as a significant risk factor for atrial fibrosis. Atrial pressure-related indices were linearly correlated with atrial fibrosis. Compared to wild-type mice, CS-CREM heterozygous mice exhibited significantly higher atrial pressure and aggravated atrial fibrosis. Single-nucleus sequencing revealed that atrial endocardial endothelial cells in CS-CREM mice underwent endothelial-mesenchymal transition (EnMT) into fibroblasts, with mechanical stress protein Flna being a critical regulatory protein. In vitro experiments demonstrated mechanical stretch-induced EnMT in ACCE and HUVEC cell lines. Mechanical stretch-activated mechanosensitive receptors on ACCE cell membranes led to increased intracellular calcium levels and calpain activation, which cleaved Flna into Flna 90. Flna 90 facilitated the nuclear translocation of transcription factor Smad3/7 and TGF-β, promoting the expressions of EnMT genes. This EnMT process was reversible with Mg101. In vivo experiments showed that Mg101 reduced the incidence of AF and mitigated atrial fibrosis in CS-CREM mice.
Conclusion: Mechanical stress induces cleaved Flna 90 from Flna in atrial endocardial endothelial cells, thus assisting transcription factors Smad3/7 and TGF-β in nuclear translocation, regulating EnMT and mediating atrial fibrosis.
Methods: Patients with AF who underwent radiofrequency ablation were recruited. Clinical data, including high-density mapping and imaging information, was analyzed. Multivariate regression analysis was performed to identify risk factors for low-voltage areas in the atrium. The CS-CREM mouse model, an autonomic AF model, was previously developed by our research group. Millar pressure catheters were used to measure left ventricular, right ventricular, and right atrial pressures in CS-CREM mice. Single-nucleus sequencing was employed to map the single-cell transcriptomes of atrial samples in CS-CREM and wild-type mice at different disease stages. Human primary atrial endocardial endothelial cells (ACCE) and HUVEC cell lines were subjected to mechanical stretch using the Flexcell tension system, followed by in vitro validation experiments. Mg101, a calpain inhibitor, was administered to CS-CREM mice for in vivo validation experiments.
Results: Elevated atrial pressure in AF patients was identified as a significant risk factor for atrial fibrosis. Atrial pressure-related indices were linearly correlated with atrial fibrosis. Compared to wild-type mice, CS-CREM heterozygous mice exhibited significantly higher atrial pressure and aggravated atrial fibrosis. Single-nucleus sequencing revealed that atrial endocardial endothelial cells in CS-CREM mice underwent endothelial-mesenchymal transition (EnMT) into fibroblasts, with mechanical stress protein Flna being a critical regulatory protein. In vitro experiments demonstrated mechanical stretch-induced EnMT in ACCE and HUVEC cell lines. Mechanical stretch-activated mechanosensitive receptors on ACCE cell membranes led to increased intracellular calcium levels and calpain activation, which cleaved Flna into Flna 90. Flna 90 facilitated the nuclear translocation of transcription factor Smad3/7 and TGF-β, promoting the expressions of EnMT genes. This EnMT process was reversible with Mg101. In vivo experiments showed that Mg101 reduced the incidence of AF and mitigated atrial fibrosis in CS-CREM mice.
Conclusion: Mechanical stress induces cleaved Flna 90 from Flna in atrial endocardial endothelial cells, thus assisting transcription factors Smad3/7 and TGF-β in nuclear translocation, regulating EnMT and mediating atrial fibrosis.
More abstracts on this topic:
Sotagliflozin Reduces Contractile Work In Human Living Myocardial Slices
Taichman Rebecca, Smolyak Julia, Bedi Kenneth, Patel Sapna, Margulies Kenneth, Day Sharlene, Lee Benjamin
Aligned Mechanical Tension Improves Electrophysiology of iPSC-Derived Engineered Heart TissueRamahdita Ghiska, Chau Thao, Bhakta Riya, Pobee Charlene, Jiang Huanzhu, Genin Guy, Huebsch Nathaniel