Final ID: Sa4092
Association of transcatheter edge-to-edge repair with clinical outcomes in atrial functional mitral regurgitation
Abstract Body (Do not enter title and authors here): Background: Atrial functional mitral regurgitation (AFMR) constitutes ~25% of ≥moderate MR and has been associated with a high prevalence and incidence of HFpEF. Transcatheter edge-to-edge repair (TEER) has shown effectiveness in improving symptoms in AFMR, but its impact on mortality and HF hospitalizations is unknown.
Objective: Evaluate rates of mortality and HF hospitalizations in patients with ≥moderate AFMR treated with TEER and compare them to conservative management.
Methods: Consecutive adults with ≥moderate AFMR (functional MR with left atrial enlargement, ejection fraction≥50%, and absent >mild left ventricular enlargement) were identified retrospectively. Ecxlusion criteria were previous cardiac surgery, ≥moderate aortic regurgitation/stenosis, infiltrative/hypertrophic cardiomyopathy, regional wall motion abnormalities or malignancy. Propensity score matching was performed (1:1 ratio) between patients treated with TEER and patients treated conservatively based on age, sex, EuroScore II, effective regurgitant orifice area, and the integrative MR severity. To ensure similar technique in evaluation in the 2 groups, variables were obtained from the closest transthoracic echocardiogram before TEER in the TEER group [median 65 (IQR 29-111) days].
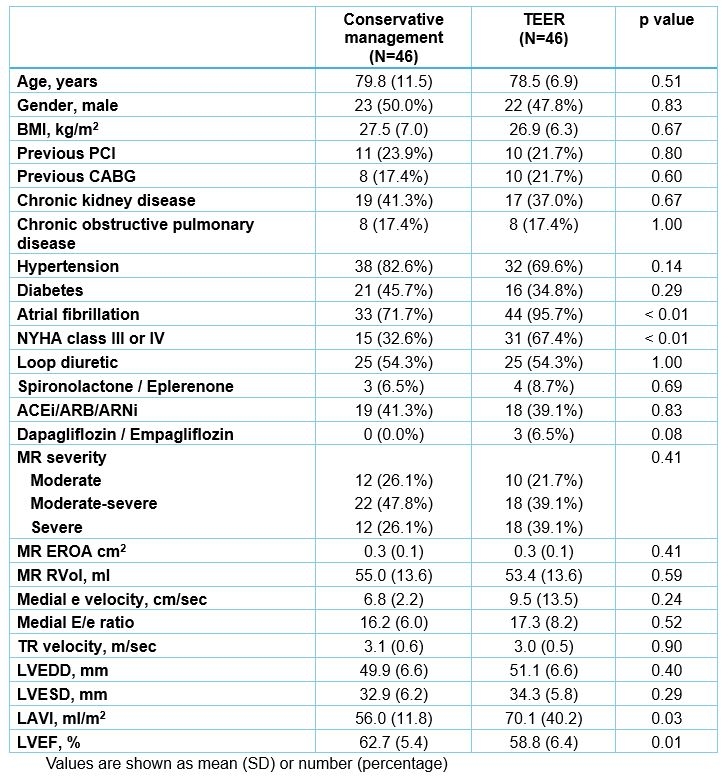
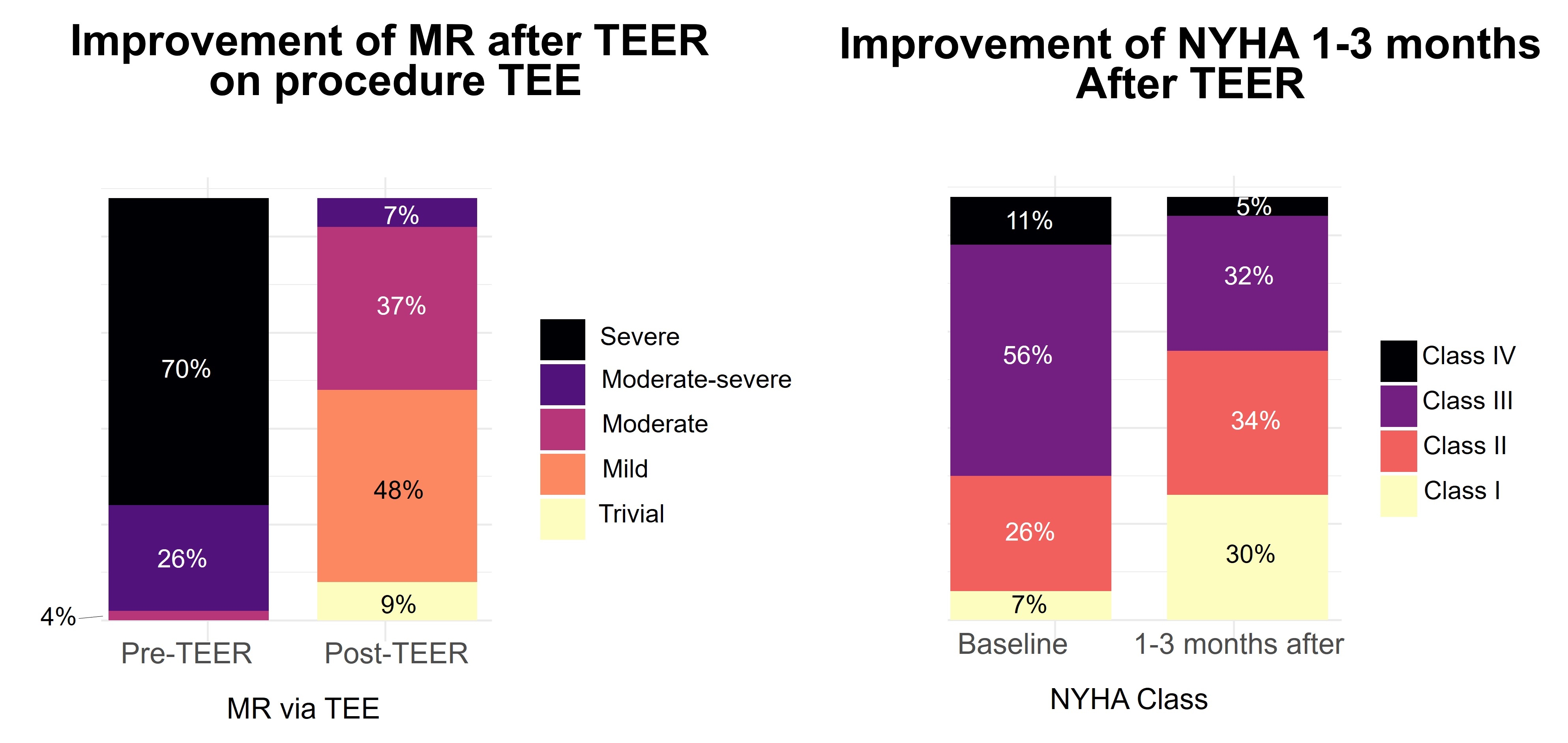
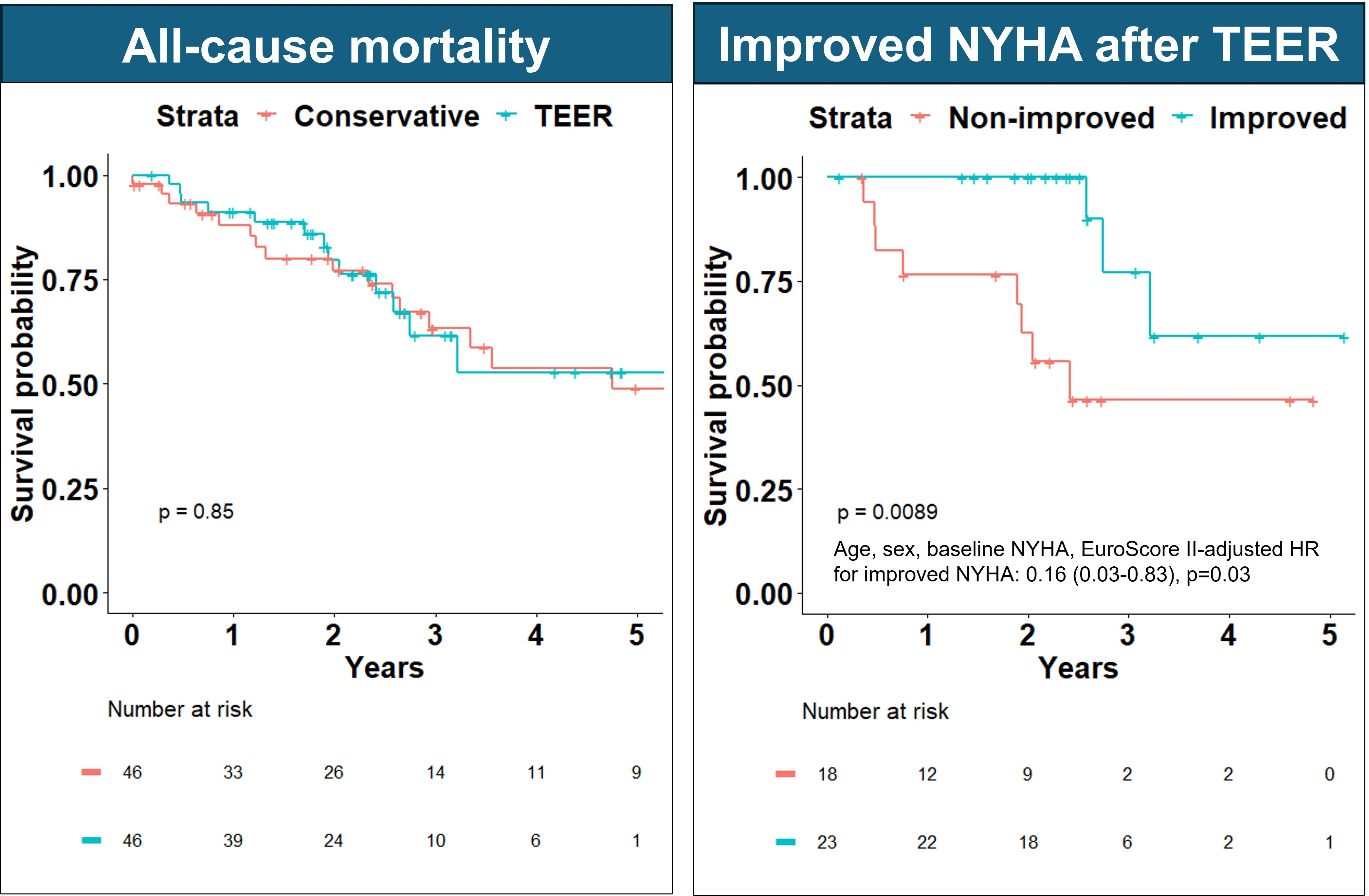
Results: Overall, 46 patients with ≥moderate AFMR treated by TEER and 46 treated conservatively were included, Table 1. Patients undergoing TEER had more frequent atrial fibrillation and NYHA class ≥III. MR improved to ≤moderate in 93% of patients undergoing TEER on procedural transesophageal echocardiography, Figure 1. On 1–3-month post-TEER follow-up, 64% had NYHA class I/II (vs 33% pre-TEER), Figure 1. Over median 2.2 (IQR 1.2-3.1) years, 32 patients died (rate 13%/year in each group, log-rank p=0.85), Figure 2. When adjusted for baseline NYHA, rates of HF hospitalizations were not different between the groups (Wald-test p-value=0.48). Residual ≥moderate AFMR in the TEER group was not associated with mortality (log-rank p=0.83), but ≥1 class improvement of NYHA in patients with baseline NYHA >1 was associated with decreased mortality, Figure 2.
Conclusion: MR severity and NYHA class improved with TEER for AFMR, and improved NYHA class was associated with decreased mortality. However, the association of TEER with decreased risk of mortality or HF hospitalization remains unproven, and larger randomized studies are needed to evaluate the potential role of TEER on these outcomes in AFMR.
Objective: Evaluate rates of mortality and HF hospitalizations in patients with ≥moderate AFMR treated with TEER and compare them to conservative management.
Methods: Consecutive adults with ≥moderate AFMR (functional MR with left atrial enlargement, ejection fraction≥50%, and absent >mild left ventricular enlargement) were identified retrospectively. Ecxlusion criteria were previous cardiac surgery, ≥moderate aortic regurgitation/stenosis, infiltrative/hypertrophic cardiomyopathy, regional wall motion abnormalities or malignancy. Propensity score matching was performed (1:1 ratio) between patients treated with TEER and patients treated conservatively based on age, sex, EuroScore II, effective regurgitant orifice area, and the integrative MR severity. To ensure similar technique in evaluation in the 2 groups, variables were obtained from the closest transthoracic echocardiogram before TEER in the TEER group [median 65 (IQR 29-111) days].
Results: Overall, 46 patients with ≥moderate AFMR treated by TEER and 46 treated conservatively were included, Table 1. Patients undergoing TEER had more frequent atrial fibrillation and NYHA class ≥III. MR improved to ≤moderate in 93% of patients undergoing TEER on procedural transesophageal echocardiography, Figure 1. On 1–3-month post-TEER follow-up, 64% had NYHA class I/II (vs 33% pre-TEER), Figure 1. Over median 2.2 (IQR 1.2-3.1) years, 32 patients died (rate 13%/year in each group, log-rank p=0.85), Figure 2. When adjusted for baseline NYHA, rates of HF hospitalizations were not different between the groups (Wald-test p-value=0.48). Residual ≥moderate AFMR in the TEER group was not associated with mortality (log-rank p=0.83), but ≥1 class improvement of NYHA in patients with baseline NYHA >1 was associated with decreased mortality, Figure 2.
Conclusion: MR severity and NYHA class improved with TEER for AFMR, and improved NYHA class was associated with decreased mortality. However, the association of TEER with decreased risk of mortality or HF hospitalization remains unproven, and larger randomized studies are needed to evaluate the potential role of TEER on these outcomes in AFMR.
More abstracts on this topic:
Cardiac Cachexia: A Case of Esophageal Compression
Ukani Zahra, Maddali Aditya, Bandaru Mrudula, Sreedhara Karthik, Solomon Allen
Association of Systemic Inflammatory Response Syndrome with Cardiovascular Events after Mitral Transcatheter Edge-to-edge RepairMannina Carlo, Sharma Samin, Kini Annapoorna, Lerakis Stamatios, Sharma Akarsh, Carbone Andreina, Bossone Eduardo, Tuttolomondo Antonino, Argulian Edgar, Neibart Eric, Halperin Jonathan, Dangas George