Final ID: 4136785
Interpreting Population Mean Treatment Effects in the Kansas City Cardiomyopathy Questionnaire
Abstract Body (Do not enter title and authors here):
Introduction: The Kansas City Cardiomyopathy Questionnaire (KCCQ) is a common outcome in heart failure trials. While comparing means between treatment groups improves statistical power, mean effects do not necessarily reflect the clinical benefit experienced by patients.
Objectives: To evaluate the relationship between mean KCCQ treatment effects in clinical trials and the proportions of patients experiencing clinically important improvements across a range of heart failure etiologies and interventions.
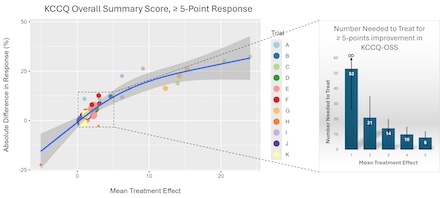
Methods: We performed a patient-level meta-analysis of 11 randomized trials (9,977 patients) to examine the relationship between mean KCCQ Overall Summary Score (OSS) treatment effects and the absolute differences in the proportions of patients experiencing clinically important (≥5 points) and moderate or larger (≥10 points) improvements. Validation was performed in 7 additional trials.
Results: Group mean KCCQ-OSS differences were strongly correlated with absolute differences in clinically important changes (Spearman correlations 0.76-0.92). For example, a mean KCCQ-OSS treatment effect of 2.5 points (half of a minimally important difference for an individual patient) was associated with an absolute difference of 6.0% (95% prediction interval [PI] 4.0-8.1) in the proportion of patients improving ≥5 points and 5.0% (95% PI 3.1-7.0) in the proportion improving ≥10 points, corresponding to NNTs of 17 (95% PI 12-25) and 20 (95% PI 14-33), respectively. Similar relationships were observed in the validation studies.
Conclusions: Inferences on clinical impact based on population-level mean treatment effects are misleading since even small between group differences may reflect clinically important treatment benefits at the patient level. Clinical trials should explicitly describe the distributions of patient-level KCCQ changes to support clinical interpretation of their results.
Introduction: The Kansas City Cardiomyopathy Questionnaire (KCCQ) is a common outcome in heart failure trials. While comparing means between treatment groups improves statistical power, mean effects do not necessarily reflect the clinical benefit experienced by patients.
Objectives: To evaluate the relationship between mean KCCQ treatment effects in clinical trials and the proportions of patients experiencing clinically important improvements across a range of heart failure etiologies and interventions.
Methods: We performed a patient-level meta-analysis of 11 randomized trials (9,977 patients) to examine the relationship between mean KCCQ Overall Summary Score (OSS) treatment effects and the absolute differences in the proportions of patients experiencing clinically important (≥5 points) and moderate or larger (≥10 points) improvements. Validation was performed in 7 additional trials.
Results: Group mean KCCQ-OSS differences were strongly correlated with absolute differences in clinically important changes (Spearman correlations 0.76-0.92). For example, a mean KCCQ-OSS treatment effect of 2.5 points (half of a minimally important difference for an individual patient) was associated with an absolute difference of 6.0% (95% prediction interval [PI] 4.0-8.1) in the proportion of patients improving ≥5 points and 5.0% (95% PI 3.1-7.0) in the proportion improving ≥10 points, corresponding to NNTs of 17 (95% PI 12-25) and 20 (95% PI 14-33), respectively. Similar relationships were observed in the validation studies.
Conclusions: Inferences on clinical impact based on population-level mean treatment effects are misleading since even small between group differences may reflect clinically important treatment benefits at the patient level. Clinical trials should explicitly describe the distributions of patient-level KCCQ changes to support clinical interpretation of their results.
More abstracts on this topic:
5-oxoproline/ OPLAH Axis Alleviates Doxorubicin-induced Cardiomyopathy By Inhibiting Ferroptosis
Jiang Meng, Guo Xinning
6-Nitrodopamine potentiates the positive chronotopic and inotropic effect induced by noradrenaline in the rat isolated heartLima Antonio, Sobanski Joao Fernando, Antunes Edson, De Nucci Gilberto