Final ID: MDP744
Efficacy and safety of aprocitentan in patients with resistant hypertension receiving at least 4 antihypertensive medications including beta (β) blockers
Abstract Body (Do not enter title and authors here): Background
Aprocitentan, a dual ETA/ETB endothelin receptor antagonist, was recently FDA-approved for use in combination with other drugs for the treatment of hypertension not adequately controlled on other antihypertensive drugs. β-blockers are used for the prevention of cardiovascular events and have antihypertensive effects. All subjects in the PRECISION study were hypertensive at screening despite receiving ≥3 antihypertensives, with 63.5% on ≥4 and 16.8% on ≥5 antihypertensives. All were switched to a standardized fixed dose triple combination of amlodipine/valsartan/hydrochlorothiazide; and the 456 patients (62.5% of the 730 total) who were on β-blockers at screening continued throughout the study as well. Of these, 173 (37.9%) had a history of ischemic heart disease and 106 (23.2%) a history of congestive heart failure, vs 52 (19%) and 37 (13.5%) not receiving β-blockers. This preplanned subgroup analysis assessed the efficacy and safety of aprocitentan in patients with resistant hypertension on the triple combination with or without β-blockers.
Methods
Changes from baseline (BL) in office systolic blood pressure (SBP) and diastolic BP (DBP) were assessed at Week 4, the end of the double-blind treatment phase with aprocitentan 12.5 mg or 25 mg per day or placebo, and at Week 36, at the end of the single-blind aprocitentan 25 mg treatment phase.
Results
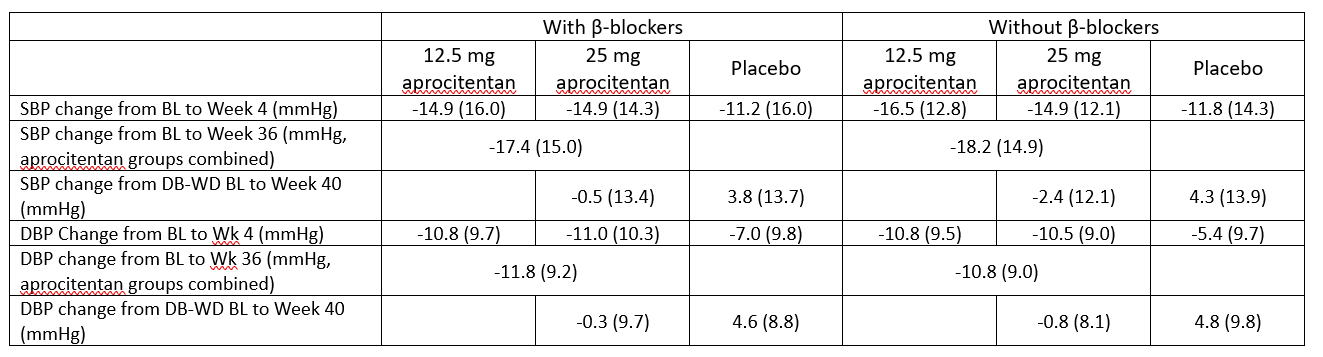
There was no difference in the efficacy of aprocitentan when patients were on a β-blocker or not. In patients on β-blockers, 12.5 mg and 25 mg aprocitentan decreased SBP from BL to Week 4 by mean -14.9 mmHg and -14.9 mmHg, respectively, vs -11.2 mmHg in the placebo group; and in patients without β-blockers by -16.5 mmHg and -14.9 mmHg vs -11.8 mmHg. Similar results were seen at Week 36, as compared with BL for both subgroups, and at Week 4 for DBP (Table 1).
In patients on β-blockers, the incidence of edema/fluid retention at Week 4 was 9.9%, 19.3% and 2.8% in the aprocitentan 12.5 mg, 25 mg and placebo groups, respectively, as compared with 7.6%, 16.7%, and 1.0% in patients without β-blockers. In both subgroups, most events occurred during the first 8 weeks of treatment. The incidence of edema/fluid retention with aprocitentan was comparable with that of the placebo group in both subgroups thereafter.
Conclusions
Aprocitentan is effective in lowering BP and has a good safety and tolerability profile in patients with resistant hypertension who are taking 4 or more antihypertensive drugs including β-blockers.
Aprocitentan, a dual ETA/ETB endothelin receptor antagonist, was recently FDA-approved for use in combination with other drugs for the treatment of hypertension not adequately controlled on other antihypertensive drugs. β-blockers are used for the prevention of cardiovascular events and have antihypertensive effects. All subjects in the PRECISION study were hypertensive at screening despite receiving ≥3 antihypertensives, with 63.5% on ≥4 and 16.8% on ≥5 antihypertensives. All were switched to a standardized fixed dose triple combination of amlodipine/valsartan/hydrochlorothiazide; and the 456 patients (62.5% of the 730 total) who were on β-blockers at screening continued throughout the study as well. Of these, 173 (37.9%) had a history of ischemic heart disease and 106 (23.2%) a history of congestive heart failure, vs 52 (19%) and 37 (13.5%) not receiving β-blockers. This preplanned subgroup analysis assessed the efficacy and safety of aprocitentan in patients with resistant hypertension on the triple combination with or without β-blockers.
Methods
Changes from baseline (BL) in office systolic blood pressure (SBP) and diastolic BP (DBP) were assessed at Week 4, the end of the double-blind treatment phase with aprocitentan 12.5 mg or 25 mg per day or placebo, and at Week 36, at the end of the single-blind aprocitentan 25 mg treatment phase.
Results
There was no difference in the efficacy of aprocitentan when patients were on a β-blocker or not. In patients on β-blockers, 12.5 mg and 25 mg aprocitentan decreased SBP from BL to Week 4 by mean -14.9 mmHg and -14.9 mmHg, respectively, vs -11.2 mmHg in the placebo group; and in patients without β-blockers by -16.5 mmHg and -14.9 mmHg vs -11.8 mmHg. Similar results were seen at Week 36, as compared with BL for both subgroups, and at Week 4 for DBP (Table 1).
In patients on β-blockers, the incidence of edema/fluid retention at Week 4 was 9.9%, 19.3% and 2.8% in the aprocitentan 12.5 mg, 25 mg and placebo groups, respectively, as compared with 7.6%, 16.7%, and 1.0% in patients without β-blockers. In both subgroups, most events occurred during the first 8 weeks of treatment. The incidence of edema/fluid retention with aprocitentan was comparable with that of the placebo group in both subgroups thereafter.
Conclusions
Aprocitentan is effective in lowering BP and has a good safety and tolerability profile in patients with resistant hypertension who are taking 4 or more antihypertensive drugs including β-blockers.
More abstracts on this topic:
A durable reduction in blood pressure by ultrasound renal denervation: A real-world, single center experience
King Jordan, Gharib Wissam
Endothelin System Activation by Autoantibodies as a Mechanistic Pathway to Lupus-associated HypertensionButler Helen, Mccrorey Marice, Lacey Ryan, Semenikhina Marharyta, Palygin Oleg, Ergul Adviye, Oates Jim, Van Beusecum Justin