Final ID: MDP1312
Effect of Colchicine on the Perivascular Inflammation Index of Coronary Computerized Tomography Angiogram: COPIX trial
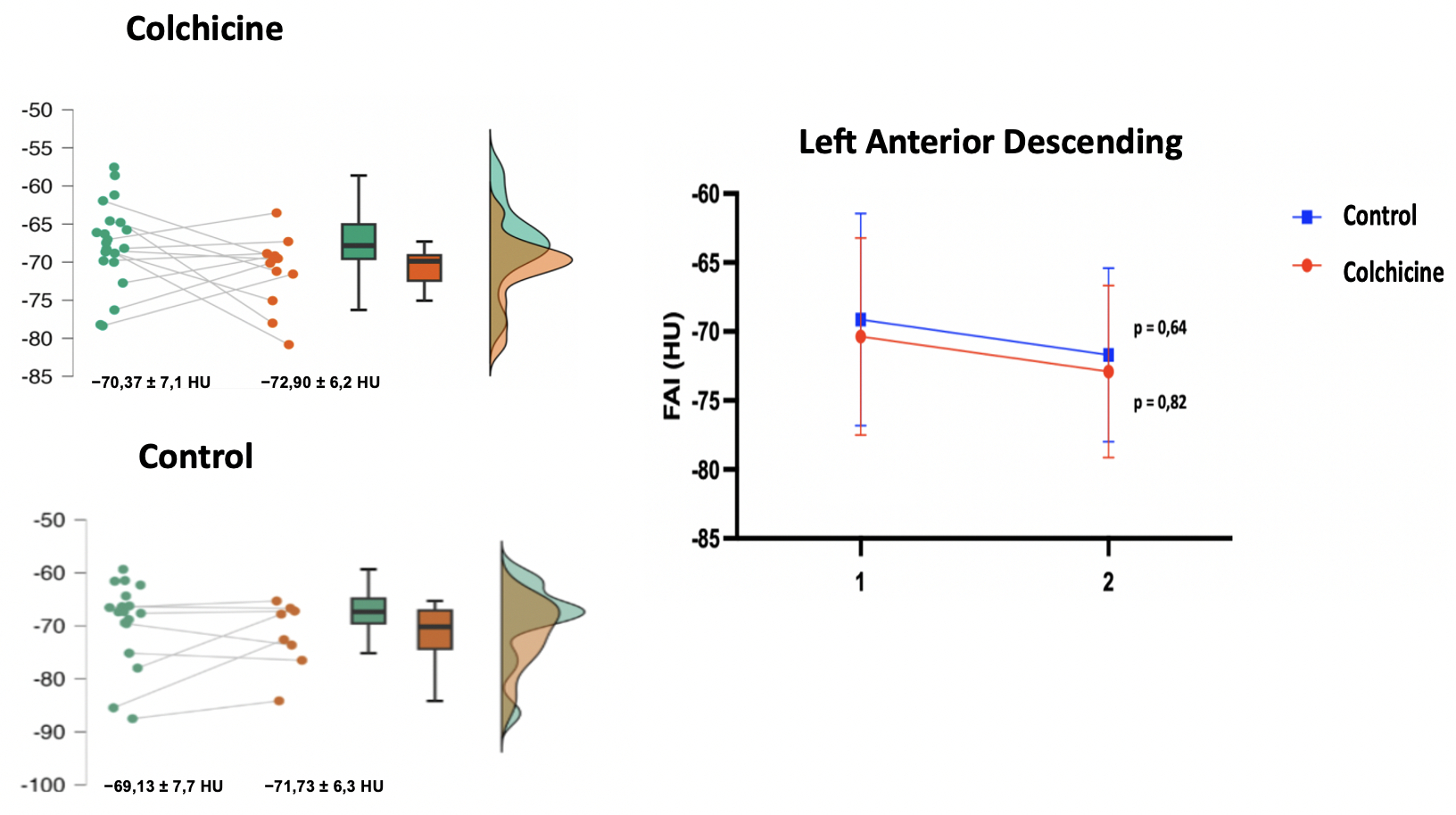
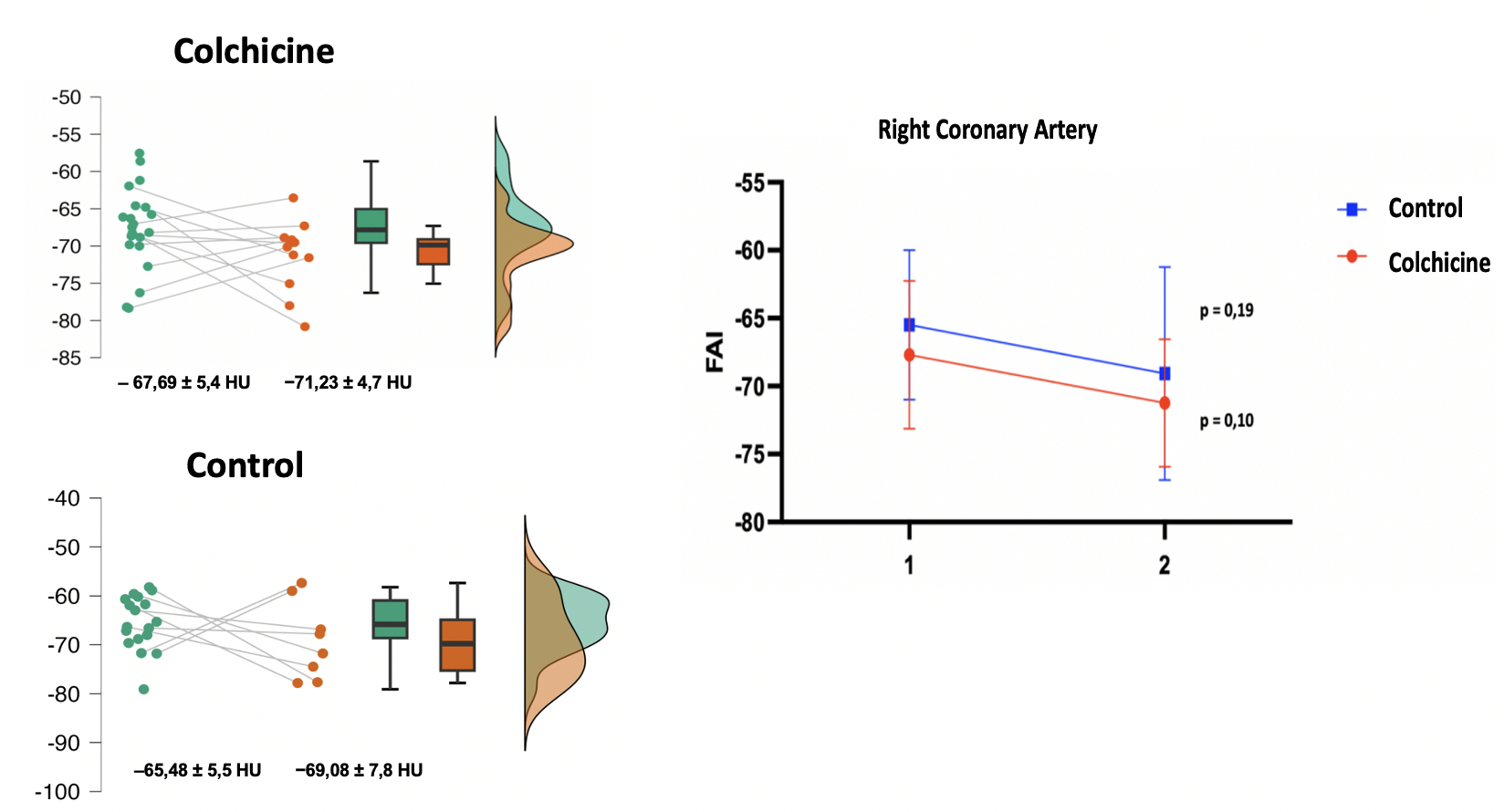
Abstract Body (Do not enter title and authors here): Inflammation is an important pillar of atherogenesis in coronary disease. Studies have documented the prognostic power of measuring the attenuation of coronary perivascular adipose tissue (PVAT) and its good correlation as an early inflammatory biomarker in the atherogenesis process. Colchicine, a medication with well-documented anti-inflammatory action and with an impact on reducing cardiovascular outcomes, may influence reducing FAI (Fat Attenuation index) assessed by coronary tomography angiography. This study aims to evaluate the effect of colchicine in reducing coronary inflammation after 12 months of using the medication. Methods: Single- center, randomized, prospective, open, ongoing study, carried out at the Heart Institute of the University of São Paulo (InCor HCFMUSP). Forty patients with documentation of FAI ≥ 70 HU in the right coronary artery (RCA) and/or left descending artery (LAD) were randomized to receive colchicine 0.5 mg/day for a period of 12 months. The primary outcome was the quantification of FAI in both therapeutic groups (colchicine and control) after a 12-month follow-up. As a secondary outcome, we evaluated the variation in total atheroma volume, calcium score, progression or regression of high plaque findings, variation low attenuation plaque volume and clinical outcomes. Results: Forty patients were included between May 2021 to April 2023, of which 22 were in the Colchicine group and 18 in the Control group. As a primary outcome, no FAI improvement in RCA and LAD was documented after a 12-month follow-up. In the Colchicine group, the mean FAI in initial LAD of −70.37 ± 7.1 HU improved to −72.90 ± 6.2 HU, but without statistical significance (p = 0.82) and Control group −69,13 ± 7,7 HU to −71,73 ± 6,3 HU (p= 0,64). FAI RCA from − 67.69 ± 5.4 HU to −71.23 ± 4.7 HU, without significance (p = 0.10) in Colchicine group and – 65,48 ± 5,5 HU to −69,08 ± 7,8 HU (p = 0,19) in control group. Conclusion: To date, a total of 40 patients have been randomized. In this interim analysis, with 50% of the sample already undergoing control coronary CT angiography, no association was observed with the use of colchicine and improvement in coronary inflammation after 12 months of using the medication.
More abstracts on this topic:
Association between ipsilateral stroke and non-stenotic (<50%) carotid disease – Analysis from the Alteplase compared to Tenecteplase Trial
Ignacio Katrina, Tkach Aleksander, Sajobi Tolulope, Buck Brian, Menon Bijoy, Almekhlafi Mohammed, Ganesh Aravind, Singh Nishita, Nagendra Shashank, Bala Fouzi, Alhabli Ibrahim, Baguley Elizabeth, Poulin Therese, Sjonnesen Kirsten, Swartz Richard, Catanese Luciana
Arrhythmia and Left Ventricular Functional Recovery in Arrhythmic Myocarditis Following Immunosuppressive TherapyChelikam Nikhila, Katapadi Aashish, Darden Douglas, Gopinathannair Rakesh, Kabra Rajesh, Pothineni Naga Venkata, Atkins Donita, Lakkireddy Dhanunjaya