Final ID: 4116949
Specialized Pericyte Subtypes in the Pulmonary Capillary
Abstract Body (Do not enter title and authors here): Introduction: Lung pericytes (PCs) are mural cells in close contact with endothelial cells (ECs) in the microvasculature. A significant challenge in investigating PC biology is largely due to the absence of a unique cell marker, making it difficult to distinguish them from other mural cell populations. The identification of such a marker would allow investigators to describe the role of PCs in various diseases including pulmonary arterial hypertension.
Hypothesis: We hypothesize that HIG1 hypoxia-inducible domain family member 1B (Higd1b) is exclusively expressed in lung PCs, which contributes to hypoxia (Hx)-induced vascular remodeling.
Methods: We utilized single-cell RNA sequence (scRNA-seq) databases, spatial transcriptomics, and RNAscope from human and murine lungs to identify a PC-specific cell marker and compare the gene expression between PC subtypes. We utilized Cre-LoxP and CRISPR technology to construct a novel tamoxifen-inducible Higd1b-CreERT2 knock-in mouse model and performed lineage-tracing studies to describe the role of PC subtypes in Hx-induced pulmonary hypertension (PH).
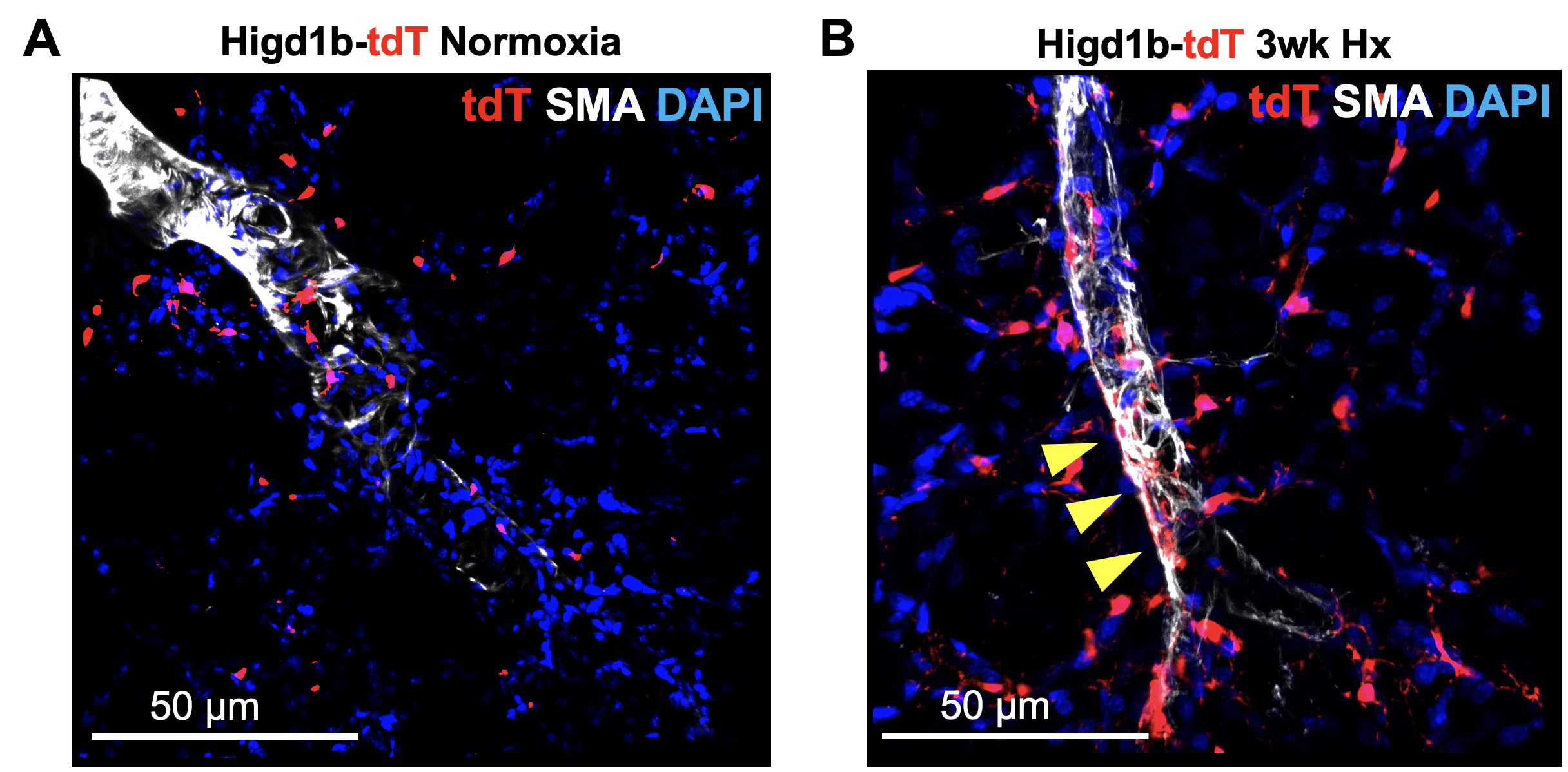
Results: ScRNA-seq analysis from the lungs of humans and mice identified Higd1b as a specific gene marker for PCs whose expression is absent in other mural cell populations. Validation with a reporter mouse line Higd1b-CreERT2::R26-tdT confirmed Higd1b-Cre+ cells specifically label PCs and no other mural cells (Fig 1A). Lineage tracing in the Hx-induced murine model of PH demonstrated the accumulation of PCs in the muscularized distal arterioles (Fig 1B). Through scRNA-seq and immunofluorescence validation, we identified two HIGD1B+ PC subtypes that exist in the pulmonary capillary: Type 1 PCs, which are quiescent in the capillaries, and Type 2 PCs exhibit multipotent cell-like properties and accumulate in the arterioles after exposure to Hx. Furthermore, we found Type 2 PCs transited into SMC-like cells via the upregulation of Vimentin, contributing to vascular remodeling in Hx-induced PH.
Conclusion: We identified Higd1b as a unique marker for PCs and generated a novel Higd1b-CreERT2 mouse that specifically labels PCs in the lungs. The discovery of PC-subtype specialization advances our understanding of lung pericyte biology and PC’s contribution to capillary remodeling under pathological conditions.
Hypothesis: We hypothesize that HIG1 hypoxia-inducible domain family member 1B (Higd1b) is exclusively expressed in lung PCs, which contributes to hypoxia (Hx)-induced vascular remodeling.
Methods: We utilized single-cell RNA sequence (scRNA-seq) databases, spatial transcriptomics, and RNAscope from human and murine lungs to identify a PC-specific cell marker and compare the gene expression between PC subtypes. We utilized Cre-LoxP and CRISPR technology to construct a novel tamoxifen-inducible Higd1b-CreERT2 knock-in mouse model and performed lineage-tracing studies to describe the role of PC subtypes in Hx-induced pulmonary hypertension (PH).
Results: ScRNA-seq analysis from the lungs of humans and mice identified Higd1b as a specific gene marker for PCs whose expression is absent in other mural cell populations. Validation with a reporter mouse line Higd1b-CreERT2::R26-tdT confirmed Higd1b-Cre+ cells specifically label PCs and no other mural cells (Fig 1A). Lineage tracing in the Hx-induced murine model of PH demonstrated the accumulation of PCs in the muscularized distal arterioles (Fig 1B). Through scRNA-seq and immunofluorescence validation, we identified two HIGD1B+ PC subtypes that exist in the pulmonary capillary: Type 1 PCs, which are quiescent in the capillaries, and Type 2 PCs exhibit multipotent cell-like properties and accumulate in the arterioles after exposure to Hx. Furthermore, we found Type 2 PCs transited into SMC-like cells via the upregulation of Vimentin, contributing to vascular remodeling in Hx-induced PH.
Conclusion: We identified Higd1b as a unique marker for PCs and generated a novel Higd1b-CreERT2 mouse that specifically labels PCs in the lungs. The discovery of PC-subtype specialization advances our understanding of lung pericyte biology and PC’s contribution to capillary remodeling under pathological conditions.
More abstracts on this topic:
A Therapeutic Vaccine Against Transforming Growth Factor-β Prevents Aortic Aneurysm Formation in a Mouse Model of Marfan Syndrome
Okamura Shun, Yagi Hiroki, Hayashi Hiroki, Ueda Tomomi, Shindo Akito, Kadowaki Hiroshi, Nakagami Hironori, Takeda Norihiko, Akazawa Hiroshi
Cell physiology and multi-omics analysis revealed disordered embryogenesis as early as mesoderm specification in models of Holt-Oram SyndromeSatoh Masahiro, Zu Yao, Kc Ashmita, Beerens Manu, Macrae Calum, Teramoto Ryota, Victorio Rachelle, Goto Shinichi, Zhu Wandi, Homilius Max, Kaveh Aryan, Chiang David, Saha Kusumika