Final ID: MDP506
High Intensity Statin Use Improved ASCVD Outcomes in Patients with Severe Hyperlipidemia in a Primary Prevention Cohort
Abstract Body (Do not enter title and authors here):
Background
High intensity statin therapy is currently recommended for primary prevention of ASCVD in patients with severe hyperlipidemia (HLD), defined as low-density lipoprotein cholesterol (LDL-C) of ≥190 mg/dL. We examined statin use for patients with severe HLD and associated ASCVD outcomes in a large contemporary healthcare network.
Methods
Using EMR data, patients between the ages of 20 and 79 with a lipid bloodwork result between 2013 and 2017 without ASCVD by ICD9 and ICD10 codes were identified. Patients with LDL-C ≥ 190 mg/dL were identified. Statin use was stratified by no statin, guideline directed statin intensity (GDSI), and less than guideline directed statin intensity (
Results
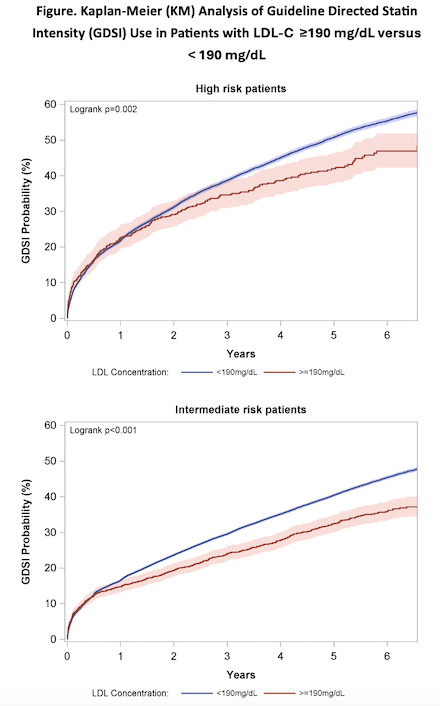
Out of 282,298 primary prevention patients, a total of 5,205 (1.8%) had LDL-C ≥ 190 mg/dL. These patients were 55.6% female and had an average age of 52.5 ± 12.2. At first encounter, 3.7% were on GDSI. Over a 5-year follow up, KM probabilities revealed 42% of high-risk patients with severe HLD not initially on GDSI would achieve new GDSI use. Compared to patients on GDSI, those on no statin therapy were at significantly higher risk of MI (HR = 2.36, 95% CI [1.38 – 4.04]) and stroke/TIA (HR= 2.70, 95% CI [1.43 – 5.09]); both p < 0.05. There was no significant difference in risk of all-cause mortality (HR = 1.41, 95% CI [0.90 – 2.21]). Patients on
Conclusion
Among primary prevention patients with severe HLD, high intensity statins are underutilized. Prevalence of this suboptimal use of statins is linked to greater risk of ASCVD events. Healthcare system-wide interventions can identify measures to assess burden of high-risk patients with severe HLD and implement lipid-lowering therapies optimally.
Background
High intensity statin therapy is currently recommended for primary prevention of ASCVD in patients with severe hyperlipidemia (HLD), defined as low-density lipoprotein cholesterol (LDL-C) of ≥190 mg/dL. We examined statin use for patients with severe HLD and associated ASCVD outcomes in a large contemporary healthcare network.
Methods
Using EMR data, patients between the ages of 20 and 79 with a lipid bloodwork result between 2013 and 2017 without ASCVD by ICD9 and ICD10 codes were identified. Patients with LDL-C ≥ 190 mg/dL were identified. Statin use was stratified by no statin, guideline directed statin intensity (GDSI), and less than guideline directed statin intensity (
Results
Out of 282,298 primary prevention patients, a total of 5,205 (1.8%) had LDL-C ≥ 190 mg/dL. These patients were 55.6% female and had an average age of 52.5 ± 12.2. At first encounter, 3.7% were on GDSI. Over a 5-year follow up, KM probabilities revealed 42% of high-risk patients with severe HLD not initially on GDSI would achieve new GDSI use. Compared to patients on GDSI, those on no statin therapy were at significantly higher risk of MI (HR = 2.36, 95% CI [1.38 – 4.04]) and stroke/TIA (HR= 2.70, 95% CI [1.43 – 5.09]); both p < 0.05. There was no significant difference in risk of all-cause mortality (HR = 1.41, 95% CI [0.90 – 2.21]). Patients on
Conclusion
Among primary prevention patients with severe HLD, high intensity statins are underutilized. Prevalence of this suboptimal use of statins is linked to greater risk of ASCVD events. Healthcare system-wide interventions can identify measures to assess burden of high-risk patients with severe HLD and implement lipid-lowering therapies optimally.
More abstracts on this topic:
Ability of Composite Magnetic Resonance Brain Imaging Scores to Predict Functional Outcomes in Survivors of Cardiac Arrest
Nguyen Thuhien, Town James, Wahlster Sarah, Johnson Nicholas, Poilvert Nicolas, Lin Victor, Ukatu Hope, Matin Nassim, Davis Arielle, Taylor Breana, Thomas Penelope, Sharma Monisha
A Multimodality Education Model Improves Healthcare Professionals' Competency in Managing Cardiovascular Risk Factors in Type 2 Diabetes: A Mixed-Methods StudyMadhusudhan Divya, Pressley Alyssa, El Sayed Nuha, Okeke Oge, Bradley Sarah, Blanco Caroline, Perla Esteban, Jennings Ruby, Picou Kylie, Mcweeny Patrick, Crabill Carrianne