Final ID: We0060
Novel Role of Pcpe2 in Adipocyte Differentiation and Lipoprotein Metabolism
Abstract Body:
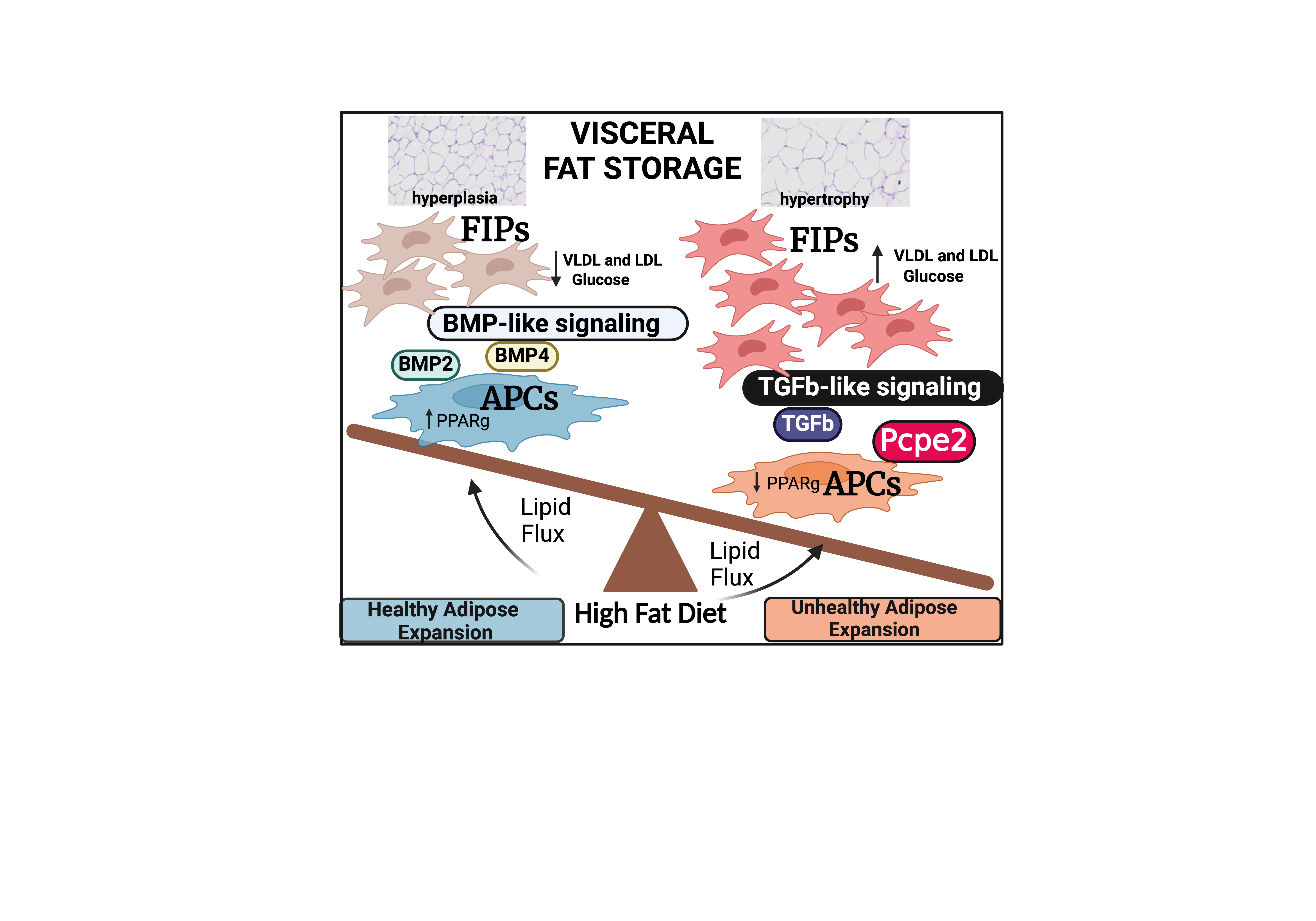
The rising prevalence of obesity in the United States continues to heighten the risk of developing type 2 diabetes and cardiovascular disease (CVD), despite the availability of numerous preventive therapies. Excessive caloric intake contributes to this epidemic by stressing adipocytes, or fat-storing cells, to deposit excess lipid, leading to hypertrophy. In contrast, healthy adipose tissue expansion, or hyperplasia, occurs when adipose tissue precursor cells (PCs) in the stromal vascular fraction (SVF) differentiate and store incoming fat in newly formed adipocytes. Single-cell RNA sequencing of visceral adipose tissue (VAT) from both mouse and human samples has identified two main types of PCs in the SVF. One type, known as adipocyte precursor cells (APCs), readily differentiate into new fat-storing adipocytes. The other, called fibroinflammatory adipocyte progenitors (FAPs), differentiate under specific conditions and primarily secrete cytokines, such as CCL2 and IL-6, which inhibit APC differentiation and promote inflammation. It is well-established that a high-fat diet (HFD) triggers several key signaling pathways, one of which is transforming growth factor beta (TGFβ). Recently, we discovered that VAT PCs express high levels of the extracellular matrix (ECM) protein procollagen C-endopeptidase enhancer 2 (Pcpe2, gene name Pcolce2), which is significantly upregulated (>4-fold) by HFD feeding and appears to play a role in TGFβ signaling. Preliminary data suggest that Pcpe2 expression stimulates TGFβ signaling, leading to an increase in FAP numbers and hypertrophic adipose tissue expansion. To investigate the mechanism of action, we created two mouse models to study Pcpe2 expression and its impact on adipose remodeling. The first is an adipose tissue (AT)-specific Pcpe2-hemagglutinin tagged (HA) over-expressor (AT-Pcpe2oxHA), and the second is an adipose-specific Pcpe2 knockout (KO) line (AT-Pcpe2KO). Preliminary findings show that AT-Pcpe2KO mice exhibit a reduction (~35-40%) in body and VAT pad weight, while AT-Pcpe2oxHA mice show a ~40-45% increase in body and VAT pad weight, characterized by hypertrophic adipocytes. Notably, AT-Pcpe2KO mice show considerable resistance to HFD-induced inflammation and hypertrophy, associated with reduced inflammation and increased APCs, while HFD fed AT-Pcpe2oxHA mice show the reverse. Overall, these findings suggest that Pcpe2 is a key driver of lipid metabolism and fat remodeling in both mice and humans.
The rising prevalence of obesity in the United States continues to heighten the risk of developing type 2 diabetes and cardiovascular disease (CVD), despite the availability of numerous preventive therapies. Excessive caloric intake contributes to this epidemic by stressing adipocytes, or fat-storing cells, to deposit excess lipid, leading to hypertrophy. In contrast, healthy adipose tissue expansion, or hyperplasia, occurs when adipose tissue precursor cells (PCs) in the stromal vascular fraction (SVF) differentiate and store incoming fat in newly formed adipocytes. Single-cell RNA sequencing of visceral adipose tissue (VAT) from both mouse and human samples has identified two main types of PCs in the SVF. One type, known as adipocyte precursor cells (APCs), readily differentiate into new fat-storing adipocytes. The other, called fibroinflammatory adipocyte progenitors (FAPs), differentiate under specific conditions and primarily secrete cytokines, such as CCL2 and IL-6, which inhibit APC differentiation and promote inflammation. It is well-established that a high-fat diet (HFD) triggers several key signaling pathways, one of which is transforming growth factor beta (TGFβ). Recently, we discovered that VAT PCs express high levels of the extracellular matrix (ECM) protein procollagen C-endopeptidase enhancer 2 (Pcpe2, gene name Pcolce2), which is significantly upregulated (>4-fold) by HFD feeding and appears to play a role in TGFβ signaling. Preliminary data suggest that Pcpe2 expression stimulates TGFβ signaling, leading to an increase in FAP numbers and hypertrophic adipose tissue expansion. To investigate the mechanism of action, we created two mouse models to study Pcpe2 expression and its impact on adipose remodeling. The first is an adipose tissue (AT)-specific Pcpe2-hemagglutinin tagged (HA) over-expressor (AT-Pcpe2oxHA), and the second is an adipose-specific Pcpe2 knockout (KO) line (AT-Pcpe2KO). Preliminary findings show that AT-Pcpe2KO mice exhibit a reduction (~35-40%) in body and VAT pad weight, while AT-Pcpe2oxHA mice show a ~40-45% increase in body and VAT pad weight, characterized by hypertrophic adipocytes. Notably, AT-Pcpe2KO mice show considerable resistance to HFD-induced inflammation and hypertrophy, associated with reduced inflammation and increased APCs, while HFD fed AT-Pcpe2oxHA mice show the reverse. Overall, these findings suggest that Pcpe2 is a key driver of lipid metabolism and fat remodeling in both mice and humans.
More abstracts on this topic:
24-hour Movement Behaviors and BMI Among a National, Diverse Sample of Adolescents
Ajibewa Tiwaloluwa, Master Lindsay, Booker Robert, Wong Mandy, Reichenberger David, Mathew Gina, Buxton Orfeu, Chang Anne-marie, Hale Lauren
Adiposity and Cardiac Function in South Asian Americans: Findings from the MASALA StudyKanaya Alka, Nelson Lauren, Running Allison, Lin Feng, Kandula Namratha, Gadgil Meghana, Win Sithu, Shah Sanjiv