Final ID: LB45
BXOS110 for Acute Ischemic Stroke Treatment (BEST): A Multicenter, Double-Blind, Randomized, Placebo-Controlled, Phase 2 Trial
Abstract Body: Background: Neuroprotective agents offer the potential for mitigating ischemic brain injury caused by excitotoxicity, oxidative stress, and inflammation. BXOS110, a novel inhibitor targeting the PSD-95 protein, disrupts the NMDAR/PSD-95/nNOS complex and has demonstrated efficacy in reducing neuronal damage in preclinical studies. Building on positive safety results from Phase 1 trials, this study aims to evaluate the efficacy and safety of BXOS110 in patients with acute ischemic stroke receiving reperfusion therapy.
Method: This multicenter, double-blind, randomized, placebo-controlled Phase 2 trial, initiated on February 7th, 2024, enrolled 300 patients presenting within 3 hours of stroke onset who received reperfusion therapy (NCT06322394). Participants were randomly assigned in a 1:1:1 ratio to receive high-dose BXOS110 (3.0 mg/kg), low-dose BXOS110 (2.0 mg/kg), or placebo. The primary outcome is the proportion of participants achieving mRS score of 0-2 at 90 days. Secondary outcomes include the proportion with mRS score of 0-2 at earlier intervals, NIHSS score changes, infarct volume variation at 24 hours, and safety endpoints, such as serious adverse events (SAEs), mortality, and withdrawal due to adverse events.
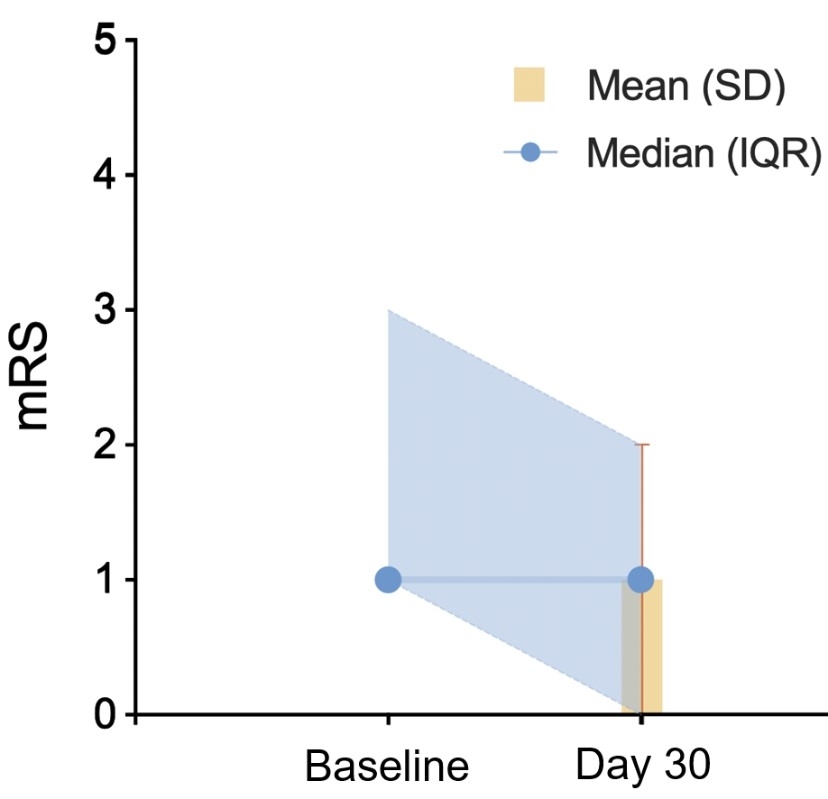
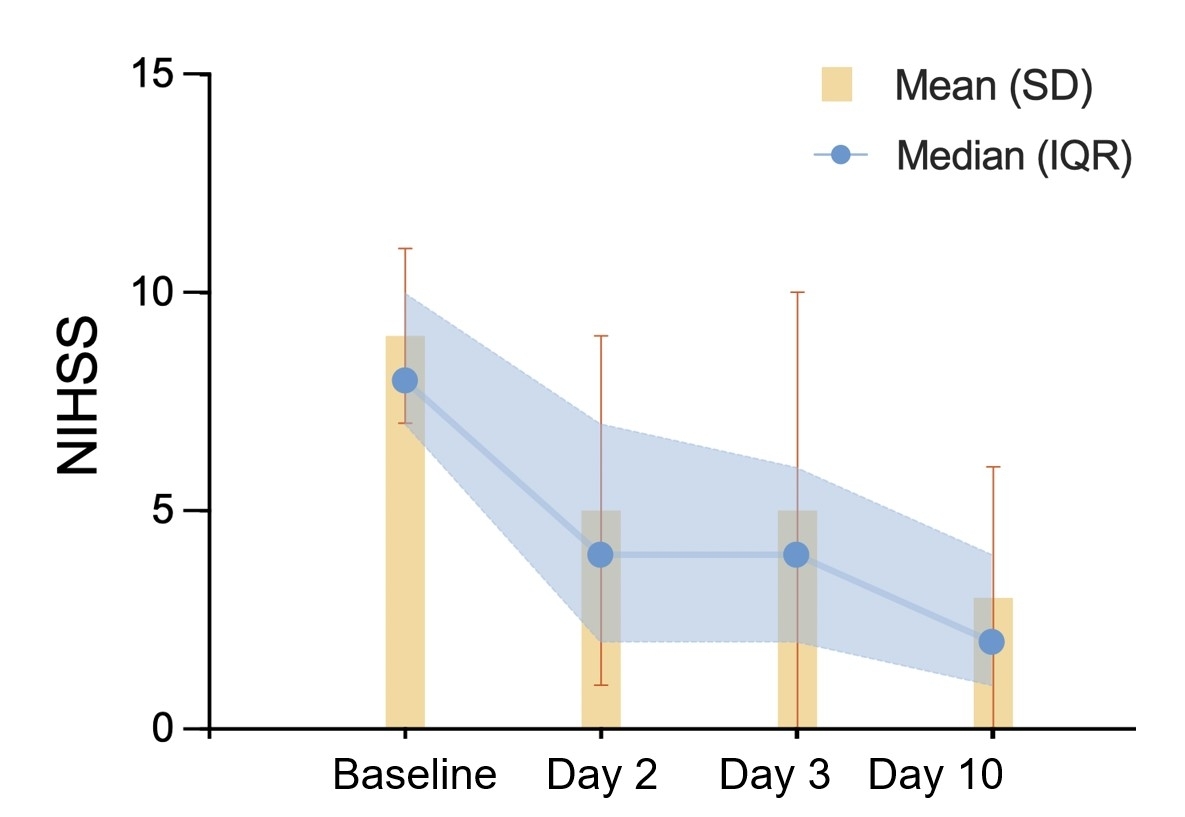
Result: The final patient enrollment was completed on August 2nd, 2024, with 100 participants assigned to each group: low-dose BXOS110, high-dose BXOS110, and placebo, according to the study protocol. The mean age of participants was 63.5±10.1 years, and 87(29%) were female. For mRS score, the pre-stroke data was mean±SD: 0±0 and median (IQR): 1 (1, 3); on Day 30 was 1±1 and 1 (0, 2); and as of October 27th, 2024, the 90-day mRS follow-up was not yet fully completed (Figure 1). The baseline NIHSS score was recorded as 9±2 and 8 (7–10). NIHSS scores on subsequent days were as follows: Day 2 was 5±4, 4 (2–7); Day 3 was 5±5, 4 (2–6); and Day 10 was 3±3, 2 (1–4) (Figure 2). Imaging evaluations were completed on September 14th, 2024, with the baseline infarct volume measured as 6.2±17.7mm3 and 24-hour infarct volume as 9.8±31.5mm3. Follow-up was 99% complete till now, with full follow-up anticipated by November 2nd, 2024. Endpoint adjudication is ongoing, and unblinding is scheduled for December 15th, 2024.
Conclusion: This trial will provide critical insights into BXOS110's potential as a neuroprotective agent in ischemic stroke combined with reperfusion treatment and will support the Phase 3 study.
Method: This multicenter, double-blind, randomized, placebo-controlled Phase 2 trial, initiated on February 7th, 2024, enrolled 300 patients presenting within 3 hours of stroke onset who received reperfusion therapy (NCT06322394). Participants were randomly assigned in a 1:1:1 ratio to receive high-dose BXOS110 (3.0 mg/kg), low-dose BXOS110 (2.0 mg/kg), or placebo. The primary outcome is the proportion of participants achieving mRS score of 0-2 at 90 days. Secondary outcomes include the proportion with mRS score of 0-2 at earlier intervals, NIHSS score changes, infarct volume variation at 24 hours, and safety endpoints, such as serious adverse events (SAEs), mortality, and withdrawal due to adverse events.
Result: The final patient enrollment was completed on August 2nd, 2024, with 100 participants assigned to each group: low-dose BXOS110, high-dose BXOS110, and placebo, according to the study protocol. The mean age of participants was 63.5±10.1 years, and 87(29%) were female. For mRS score, the pre-stroke data was mean±SD: 0±0 and median (IQR): 1 (1, 3); on Day 30 was 1±1 and 1 (0, 2); and as of October 27th, 2024, the 90-day mRS follow-up was not yet fully completed (Figure 1). The baseline NIHSS score was recorded as 9±2 and 8 (7–10). NIHSS scores on subsequent days were as follows: Day 2 was 5±4, 4 (2–7); Day 3 was 5±5, 4 (2–6); and Day 10 was 3±3, 2 (1–4) (Figure 2). Imaging evaluations were completed on September 14th, 2024, with the baseline infarct volume measured as 6.2±17.7mm3 and 24-hour infarct volume as 9.8±31.5mm3. Follow-up was 99% complete till now, with full follow-up anticipated by November 2nd, 2024. Endpoint adjudication is ongoing, and unblinding is scheduled for December 15th, 2024.
Conclusion: This trial will provide critical insights into BXOS110's potential as a neuroprotective agent in ischemic stroke combined with reperfusion treatment and will support the Phase 3 study.
More abstracts on this topic:
Blocking insulin-like growth factor 1 receptor in the gut abrogates IGF-1 mediated long-term neuroprotection in middle-aged female rats
Bake Shameena, Pickle Kaylin, Kallo Mona, Bhatt Hetvi, Sohrabji Farida
3-HKA Promotes the Vascular Remodeling after Stroke by Modulating the Activation of A1/A2 Reactive AstrocytesChen Jun-min, Shi Guang, Yu Lulu, Shan Wei, Zhang Xiangjian, Wang Qun
Readers' Comments
We encourage you to enter the discussion by posting your comments and questions below.
Presenters will be notified of your post so that they can respond as appropriate.
This discussion platform is provided to foster engagement, and simulate conversation and knowledge sharing.
You have to be authorized to post a comment. Please, Login or Signup.
Rate this abstract
(Maximum characters: 500)