Final ID: LB9
Clinical Benefit of Nerinetide at One Year in Early Window Participants Enrolled in ESCAPE-NEXT
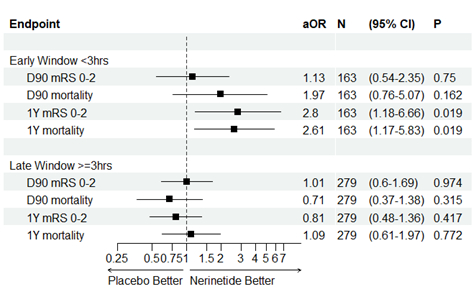
Methods: The primary outcome was functional independence (mRS 0-2) with a key secondary analysis of mortality. D90 and 1Y outcomes were evaluated using the pre-specified logistic regression analyses for the trial. Additionally, the interaction between enrollment window stratum and treatment was examined. Analyses were stratified according to whether participants were enrolled within the early or late windows.
Results: Of 850 participants, 442 completed both the D90 and 1Y assessment. The adjusted odds ratio (aOR) of achieving mRS 0-2 at 1Y for the entire cohort (n=442) were aOR 1.08 (95% CI 0.71-1.63, p=0.718). There was a statistical interaction between enrollment window and treatment (p=0.025). Among early window participants (n=163), the aOR of achieving mRS 0-2 in participants treated with nerinetide increased from 1.13 (0.54-2.35, p=0.750) at D90 to 2.80 (1.18-6.66, p=0.019) at 1Y. Additionally, the aOR for reducing mortality increased from 1.97 (0.76-5.07, p=0.162) at D90 to 2.61(1.17-5.83, p=0.019) at 1Y. No clinical benefit was observed at 1Y on mRS 0-2 or mortality in the late window participants (n=279; mRS 0-2 aOR 0.81 (0.48-1.36, p=0.417); mortality aOR 1.09 (0.61-1.97, p=0.772)).
Conclusions: Late window participants may represent slow progressors who are least likely to benefit from treatments intended to slow stroke progression. For early window ESCAPE-NEXT participants, the benefit of nerinetide was more apparent at 1Y than D90. Early window patients may represent a population that could benefit from treatment with nerinetide. Long-term benefits of neuroprotection may emerge up to a year after stroke suggesting that 1Y outcomes could be useful in stroke neuroprotection trials.
More abstracts on this topic:
Mallavarapu Monica, Kim Hyun Woo, Iyyangar Ananya, Salazar-marioni Sergio, Yoo Albert, Giancardo Luca, Sheth Sunil, Jeevarajan Jerome
A Real-World Pilot for Diagnostic Yield of Cardiac CTA vs Echocardiography in Acute Ischemic StrokeChakravarthula Nitin Ramanujam, Milani Marcus, Tessmer Megan, Staugaitis Abbey, Akimoto Kai, Markowitz Jeremy, Kalra Rajat, Nijjar Prabhjot, Streib Christopher
Readers' Comments
We encourage you to enter the discussion by posting your comments and questions below.
Presenters will be notified of your post so that they can respond as appropriate.
This discussion platform is provided to foster engagement, and simulate conversation and knowledge sharing.
You have to be authorized to post a comment. Please, Login or Signup.