Final ID: OGCTP33
Assessing the impact of PCSK9 inhibition on carotid or vertebral plaque phenotype with high resolution imaging
Abstract Body: Background: Proprotein convertase subtilisin kexin type 9 inhibitors (PCSK9i) combined with statin has enabled the achievement of low LDL-C levels and stabilizing plaque in the coronary atherosclerotic heart disease. No randomized data are available regarding the efficacy of single PCSK9i in stabilizing carotid or vertebral plaques and stroke prevention, compared with best tolerated statin therapy.
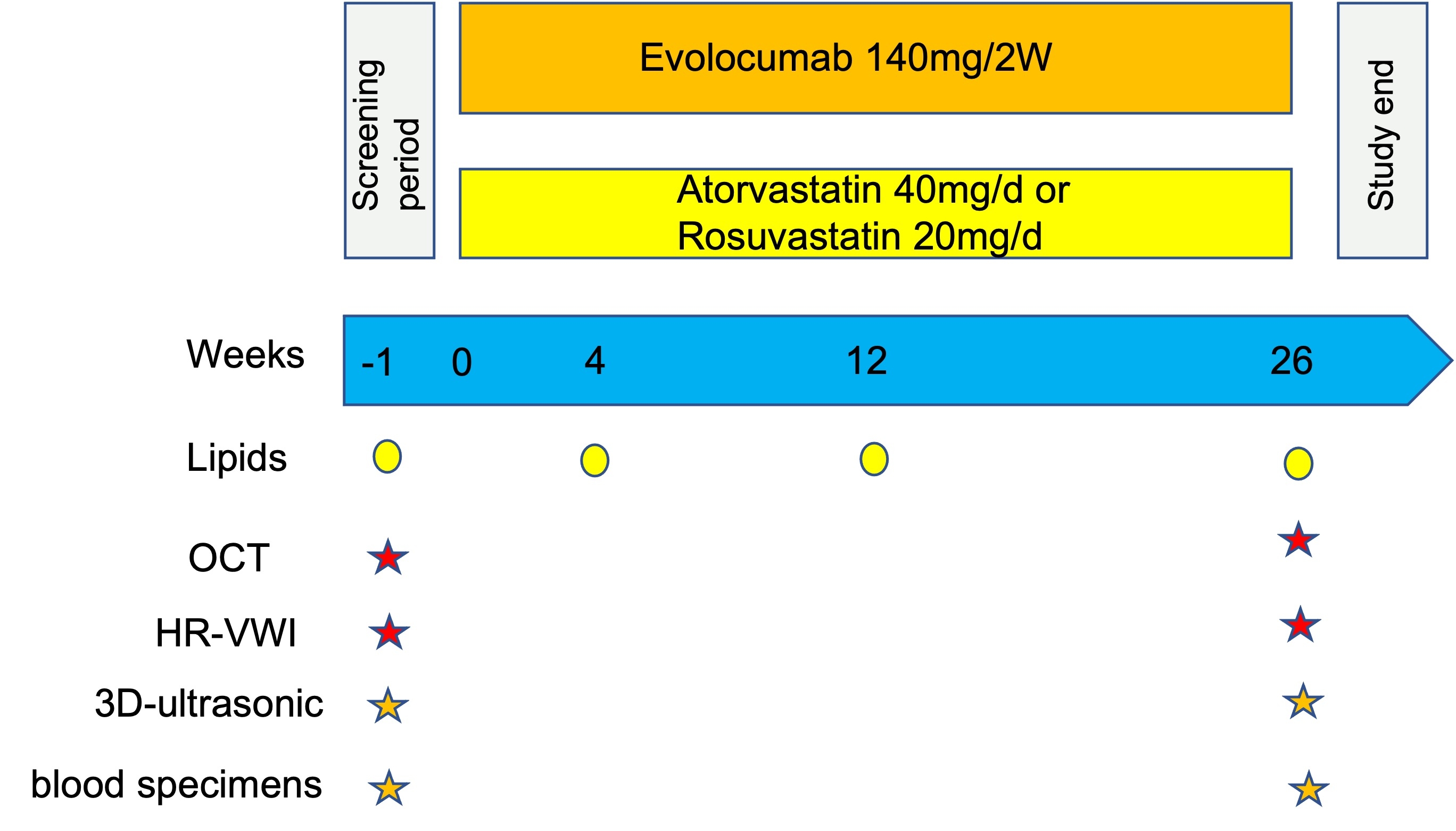
Objective: The aim of the study is to observe the impact of single evolocumab treatment for 26 weeks on carotid or vertebral artery plaque phenotype and burden, evaluated by optical coherence tomography (OCT), high-resolution vessel wall imaging (HR-VWI) and three-dimensional ultrasound, as well as ischemic events, compared with intensive statin therapy.
Methods and design: The study is a prospective, randomized, multicenter, open-label, parallel-control trial in more than 10 study centers. A maximum of 200 patients will be randomized and treated for 26 weeks to compare the efficacy of evolocumab with atorvastatin or rosuvastatin in the treatment of carotid artery or vertebral plaques. Carotid or vertebral atherosclerosis patients with suspicious stenosis 20% -69% in cerebral angiography will be assessed by OCT in those who meet clinical selection criteria. HR-VWI and three-dimensional ultrasound combined with OCT determines the plaque phenotype and burden. The subjects (n=200) will be randomized 1:1 into two groups to receive injections of evolocumab 140 mg every two weeks or intensive statin therapy per day for 26 weeks. Randomized patients return for study visits every 4 weeks and are assessed for adverse and potential end point events. During follow-up visits, at weeks 4, 12, and 26 after treatment, blood is sampled for fasting lipid profile testing. All patients are to be followed up regardless of whether they are taking study drug.
Outcomes: The primary end point was the change in the FCT from baseline to the 26-week follow-up assessed by OCT. Primary safety endpoints include occurrence of all ischemic vascular events, such as TIA, acute cerebral infarction, acute myocardial infarction, death from any cause, etc.
Analysis: The main analysis method of the primary endpoint was to use the analysis of covariance (ANCOVA) model, including the treatment group and stratification factors, and the baseline FCT was the covariate.
Objective: The aim of the study is to observe the impact of single evolocumab treatment for 26 weeks on carotid or vertebral artery plaque phenotype and burden, evaluated by optical coherence tomography (OCT), high-resolution vessel wall imaging (HR-VWI) and three-dimensional ultrasound, as well as ischemic events, compared with intensive statin therapy.
Methods and design: The study is a prospective, randomized, multicenter, open-label, parallel-control trial in more than 10 study centers. A maximum of 200 patients will be randomized and treated for 26 weeks to compare the efficacy of evolocumab with atorvastatin or rosuvastatin in the treatment of carotid artery or vertebral plaques. Carotid or vertebral atherosclerosis patients with suspicious stenosis 20% -69% in cerebral angiography will be assessed by OCT in those who meet clinical selection criteria. HR-VWI and three-dimensional ultrasound combined with OCT determines the plaque phenotype and burden. The subjects (n=200) will be randomized 1:1 into two groups to receive injections of evolocumab 140 mg every two weeks or intensive statin therapy per day for 26 weeks. Randomized patients return for study visits every 4 weeks and are assessed for adverse and potential end point events. During follow-up visits, at weeks 4, 12, and 26 after treatment, blood is sampled for fasting lipid profile testing. All patients are to be followed up regardless of whether they are taking study drug.
Outcomes: The primary end point was the change in the FCT from baseline to the 26-week follow-up assessed by OCT. Primary safety endpoints include occurrence of all ischemic vascular events, such as TIA, acute cerebral infarction, acute myocardial infarction, death from any cause, etc.
Analysis: The main analysis method of the primary endpoint was to use the analysis of covariance (ANCOVA) model, including the treatment group and stratification factors, and the baseline FCT was the covariate.
More abstracts on this topic:
A Case of Possible IgG4-Related Constrictive Pericarditis Masquerading as Idiopathic Pericarditis: A Rare and Elusive Diagnosis
Nandyal Shreyas, Sharma Bharosa, Gajjar Rohan, Varma Revati, Ezegwu Olisa, Amdetison Gedion Yilma, Tottleben Jon
9p21.3 variants drive coronary calcification by suppressing statherin expressionSoheili Fariborz, Almontashiri Naif, Heydarikhorneh Niloufar, Vilmundarson Ragnar, Chen Hsiao-huei, Stewart Alexandre
Readers' Comments
We encourage you to enter the discussion by posting your comments and questions below.
Presenters will be notified of your post so that they can respond as appropriate.
This discussion platform is provided to foster engagement, and simulate conversation and knowledge sharing.
You have to be authorized to post a comment. Please, Login or Signup.
Rate this abstract
(Maximum characters: 500)